The consultation process on the New European Chemicals Regulation REACH (Registration, Evaluation and Authorisation of Chemicals) will be finished soon. The political agreement reached in the Council, as published in June 2006, will most likely not be changed fundamentally and it can be expected that the regulation will be adopted by the end of this year. It is expected that the regulation will enter into force in the spring of 2007.
With regard to dangerous substances, REACH shifts the responsibility of adequate control of risk from the manufacture and use of chemicals from authorities to the manufacturers, importers and users of the substances. The future tasks of the authorities will be not only to enforce and control the requirements but also to support companies in reaching compliance.
What kind of requirements will producers and users of adhesives have to meet? What is the timeframe and how can companies prepare for REACH? Enterprises should start now to determine their roles and related duties under REACH, as well as potential impacts on business activities in order to be in a good position when the first elements of the regulation go into effect - probably in 2008. The common position of the council is available at http://ecb.jrc.it/DOCUMENTS/REACH/REACH_PROPOSAL/COUNCIL_COMMON_POSITION_JUNE_2006/st07524.en06.pdf . This article discusses the relevant mechanisms and options for producers and users of adhesives by an example of a fictive company named GlueMix Ltd. (see sidebar).
Registration
The registration of substances is the core element of REACH. Almost all substances have to be registered by their respective manufacturer or importer in order to maintain the right to market them. In order to register a substance, information on its properties and uses has to be generated, assessed, put together in a registration dossier and submitted to the Chemicals Agency, which will be created to oversee the REACH regulation.Which Substances Have to be Registered?
Many substances are exempt from registration under REACH, e.g., radioactive substances, non-isolited intermediates and waste, and substances specified in REACH Annexes IV and V (e.g., water, CO2and many naturally ocurring substances, like sugar, fatty acids, and minerals). Polymers are exempted preliminarily.Substances that have been classified as new substances - as well as active substances falling under the directives on biocides or pesticides - are regarded as already registered. In addition, companies can make use of exemptions for product- and process-orientated research and development (PPORD).
Apart from these exemptions, substances must be registered if they are manufactured or imported in amounts above 1 ton per year (t/a) per manufacturer/importer.

When Does a Substance Need to be Registered?
The 30,000 existing substances can be registered according to the deadlines of the so-called ‘phase-in' scheme. The scheme starts with the pre-registration of the ‘phase-in' substances one year after REACH has entered into force. If a manufacturer or importer fails to pre-register a substance within that time period, he loses the right to register in the time frame of the phase-in scheme. Instead, he must submit a dossier before he markets the substance. If he does pre-register the substance, he - like all other potential registrants of the substance - becomes member of a so-called Substance Information Exchange Forum (SIEF). The SIEF aims to facilitate cooperation in the registration and exchange of data (tests and information on substance properties).Two years later, substances above 1,000 t/a and known carcinogens, mutagens or reprotoxic substances (above 1 t/a), as well as substances classified as dangerous for the environment (R50/53; above 100 t/a), have to be registered. The deadlines shown in Figure 1 depend on the registered amount and end up to 11 years after entry into force for substances below 100 t/a.
Only single substances must be registered. Substances contained in imported preparations have to be registered as separate single substances. If the same substance is contained in several different containers by one importer, he needs to sum up the amount of these substances.
The information requirements to be submitted in the registration dossier depend on the amount to be registered. In general, the technical dossier shall contain information on substance properties from tests or other sources. For substances above 10 t/a, a chemicals safety assessment (CSA) needs to be carried out. This includes a hazard assessment, which consists of deriving the classification of a substance and threshold values, below which no effect should occur, as well as an assessment of whether or not a substance fulfils the criteria of a PBT (see authorization). If the outcome of the hazard assessment is that the substance has to be classified as dangerous, an exposure assessment and risk characterization is required. The aim of the CSA is to define the conditions of use, including necessary risk management measures under which the substance can be handled safely along its entire lifecycle. The assessments steps are to be documented in the chemical safety report (CSR). The results of the CSA have to be included in the safety data sheet and communicated down the supply chain.
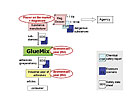
Roles and Duties under REACH
Regarding the supply chain of a chemical, REACH defines different roles implying different obligations. Looking at a simplified supply chain for adhesives in the EU, the manufacturer at the top places the substance on the market and is thus responsible for registration. If he manufactures more than 10t/a, he is to prepare a CSR, and if the substance is classified as dangerous he is to develop exposure scenarios for the uses he aims to register - such as the use in adhesives. The exposure scenarios (ES) describe the conditions of use and the necessary risk management measures that need to be taken to use the substance safely. The manufacturer is to submit the CSR (including the ES) to the agency as parts of the registration dossier. The ES should be attached to the extended safety data sheet (SDS), which is supplied to the downstream users of the substance (in this example, GlueMix). GlueMix consolidates the information in the SDSs and ESs of the components of the preparation from its supplier and forwards an SDS with attached ESs to his customers together with the preparation.GlueMix also synthesises or imports some of its raw material substances and is thus a manufacturer and importer. If GlueMix wants to continue its activities, it needs to register these substances if synthesis or import exceeds 1 t/a, just as the manufacturer at the top of the supply chain is required to do.
However, for most of his raw materials, GlueMix has the role of a DU and thus has no registration duties. The DU requirements start when GlueMix receives the first extended SDS from a supplier. GlueMix has to compare the identified use and the conditions of use described in the extended SDS and ES to its own use and use conditions. If its use is covered, GlueMix can use the supplier information to prepare the SDS and ES for the adhesive. If the information supplied to GlueMix does not cover its use and conditions of use, the company can choose between different options.
- Implement the conditions of use and recommended risk management measures (RMM) as described in the ES, consolidate, and forward the respective information to its clients;
- Communicate the deviations to the supplier and request him to reassess the use and amend the SDS and ES accordingly, if safe use can be demonstrated;
- Make his own DU-CSR (if he uses the substance or raw material in amounts above 1 t/a), notify the deviation to the agency and submit the respective safety information to its customers;
- Change the supplier to one with an ES that covers its use and conditions of use; or
- Change the raw material.
As its final goal, GlueMix must ensure that the substance is used safely in the company's own applications and that its clients receive the necessary information to use the substance safely as well.
GlueMix and its customers will have the lowest effort to comply with REACH if the SDS and ES they receive correspond to their actual uses and conditions of use in the first place. But even if the manufacturers and importers placing the substances on the market are aware of the use of their substances in adhesives, they may not have sufficient information to develop an exposure scenario with realistic assumptions, assess whether or not the use is safe, and prepare a corresponding ES.
How to Prepare for REACH
Currently, it is most important to find out how REACH will impact the individual business activities of GlueMix. First, GlueMix should screen its raw material portfolio and identify which substances it synthesizes or imports in amounts above 1 t/a and would need to be registered under REACH. For synthesized and imported substances, GlueMix should identify the registration requirements (Annexes VI to X of REACH) and collect available information on substance properties (such as its own test data or data bases). In preparing for pre-registration, GlueMix might consider whether or not to share registration with another company and what its contribution to a joint registration will be. GlueMix should also look for alternatives to registration, such as synthesized or imported substances, which may also be available on the EU market.Also, as a downstream user, GlueMix should prepare for REACH, even if the formal duties of DUs will not start until the first substances have been registered. Since the suppliers of GlueMix will have to deal with the challenges and costs of registration, market impacts can only be expected. One challenge might be substances not registered by any manufacturer or importer. This is most likely to happen for substances produced in lower volumes that have low prices, which will disappear from the market. The use in adhesives might not be covered in the CSR, or it may not be supported by the supplier, leaving the DU with the decision to make its own safety assessment for that use, or to change suppliers or raw material. Based on new test data, substances might get a new classification with consequences for the clients' risk management as well as the classification of its own product - the adhesive. In these cases, the availability of the substance is reduced or connected with an emphasis placed on communication and assessment. Early communication on these substances and/or uses may prevent unwanted surprises in the future. GlueMix should try to identify these critical substances by checking the following.
- Which substances are so-called low-volume/low-value substances (registration might not be profitable)?
- For which substances can the use as adhesive be regarded as a niche application (when the registrant is not aware of the use or is not willing to include it in the assessment)?
- Which suppliers currently do not provide sufficient information on health, safety and environmental issues? (They may not have the competence to comply with REACH.)
- Which suppliers may need support by GlueMix to prepare adequate SDS?
- Which substances have very hazardous properties?
To comply with the complex requirements of REACH, companies will find support through guidelines and help desks, which are under development. Currently, the EU Commission has launched several REACH Implementation Projects (RIPs) which, among other things, aim to develop guidance for industry to implement REACH (http://ecb.jrc.it/REACH). Among these are guidelines for the chemical safety report (RIP 3.2-2) and guidelines for downstream user requirements (RIP 3.5-2). The industry is involved in the work and contributes with comments and case studies. The European Association of Adhesive Manufacturers (FEICA) takes part in RIP 3.2-2. GlueMix should observe these activities and make use of the resulting guidelines as soon as they are available.
Authorization and Evaluation Under REACH
For some substances with very hazardous properties, an additional mechanism is foreseen by REACH to reduce potential risks. This group of substances of very high concern (SVHC) includes the following.- CMRs (carcinogenic, mutagenic or reprotoxic substances Cat. 1 and 2)
- PBTs (persistent, bioaccumulative and toxic substances) and vPvBs (very persistent and very bioaccumulative substances)
- Substances for which there is scientific evidence of an equivalent level of concern (e.g., endocrine disruptors)
These SVHC will be identified by the agency and member states, and will be listed on a ‘candidate list'. Based on this list, the agency will prioritize substances for the authorization procedure. The commissions, assisted by a committee composed of representatives of the member states (committee procedure), may decide to include candidate substances in Annex XIV of REACH. An inclusion into the Annex XIV means that, after a transitional period, the substances may not be placed on the market or used unless authorization is granted for the specific use.
Under REACH, industry will be responsible for the safe manufacture, handling and use of substances. The member state authorities are to ensure that the requirements are enforced. Another set of tasks of authorities relates to the evaluation of information, which is defined under REACH for different areas:
- Evaluation of testing proposals: Before a company carries out a vertebrate test for registration, it must submit a respective testing proposal to the agency. The agency evaluates the proposal and decides whether the proposal is adequate and whether the test is necessary.
- Evaluation of registration dossiers: A specific share of the submitted registration dossiers will be evaluated by the agency and checked with regard to compliance and plausibility.
- Evaluation of substances: Member states will choose substances regularly for in-depth risk assessment. The outcome of such an assessment may be the need for additional regulation of the substance through authorization or restriction.
This way, authorities amend the responsibilities taken by industry in cases where the potential risks require common rules and enhanced enforcement.
For more information, contact Kerstin Heitmann, SIDEBAR: GlueMix Ltd.
Medium-sized producer of adhesives
Ca. 280 products (preparations)
- Ca. 95% classified as dangerous
Ca. 300 input substances
- ca. 85% purchase in EU
- ca. 15% import from non EU-countries
- Seven substances own synthesis
- 90% classified as dangerous
