
In a continuously expanding worldwide market for pressure-sensitive adhesives (PSAs), specific trends are occurring. Environmental pressures are causing industries to turn their collective backs upon solvent technologies of the past and accept the solventless systems of the present and future. Converters initially began using hot-melt PSA systems, which offered no volatile components, accelerated process times and a dramatic reduction in cost and required space needed to operate the tunnel ovens of the past.
However, there are limitations inherent to hot-melt PSAs, such as insufficient high-temperature resistance and low chemical resistance, which may be attributed to the thermoplastic nature of the hot-melt adhesives. To combat these issues, the molecules in a PSA need to have crosslinking tendencies that bring about the necessity of energy-curable adhesives. Ultraviolet (UV) radiation energy or electron beams are used to initiate the chemical process that results in the three-dimensional crosslinked network of monomers and oligomer. These energy-curable adhesives can be either hot-melts or room-temperature liquids. RAHN USA has done extensive work studying the tendencies and developing starting point formulations for the latter two. This article examines the basics of formulating these adhesives with a specific end property in the mind of the formulator.

Components
A liquid radiation-curable PSA is comprised of four essential components - elastomer, tackifier, diluent and photoinitiator. The elastomeric in this case is a combination of monofunctional and difunctional aliphatic urethane acrylate oligomers. The high molecular weights and glass-transition temperatures (Tg) of well below ambient temperature allow the oligomer component to offer elastic-like properties at room temperature, enabling the adhesive to be extended or compressed upon pressure. Its deformability under light pressure allows it to conform to and wet out a substrate (measured as the internal tack of the system). Upon adhesive removal from that substrate, its elasticity allows it to extend greatly before separating, giving the adhesive good peel and adhesion properties.The tackifying component of this adhesive is a saturated polyester co-resin. Its function is twofold. Generally, the resin has a much lower molecular weight and higher Tg than the oligomer. This difference allows the elastomer greater mobility in the system, maximizing both its deformability under light pressure and its elastic behavior during adhesive removal. The higher glass-transition temperature of the tackifying resin brings the overall adhesive Tg to a value necessary to achieve PSA properties. Typically, a pressure-sensitive adhesive's Tg ranges from -25°C to +5°C, though this is merely a suggested range that can be altered when certain properties must be achieved, such as a high-shear-adhesion-fail temperature (SAFT) value. In general, the higher the overall Tg of an adhesive, the greater the cohesive strength and high temperature shear results, and the lower the tack and deformability properties.

Finally, the photoinitiator's role is vital as it dictates the manner of cure that the adhesive undergoes. A high surface cure photoinitiator will tend to increase shear properties, but destroy the tack of the system. A great throughcure product may leave the surface very tacky but exhibit poor cohesive strength due to the surface not being as well crosslinked, resulting in poor shear properties. A good balance of cure properties is important in maintaining proper pressure-sensitive adhesive attributes, which is what this starting-point PSA study attempts to consider.
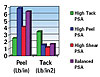
End Properties
Besides acting as diluent, the reactive monomer plays a role similar to the tackifying resin, allowing for greater deformability of the adhesive due to its low molecular weight and, depending on the monomer, adding its own flexible behavior to an adhesive. The Tgof the monomer also assists in defining the overall Tgof the adhesive system.Finally, the photoinitiator's role is vital as it dictates the manner of cure that the adhesive undergoes. A high surface cure photoinitiator will tend to increase shear properties, but destroy the tack of the system. A great throughcure product may leave the surface very tacky but exhibit poor cohesive strength due to the surface not being as well crosslinked, resulting in poor shear properties. A good balance of cure properties is important in maintaining proper pressure-sensitive adhesive attributes, which is what this starting-point PSA study attempts to consider.

Another type of shear test involves the testing of a sample under heated conditions. A Shear Adhesion Failure Test (SAFT) is a Static Shear Test administered to a PSA sample under increasing thermal conditions, typically 1°C or 2°C per minute. The recorded SAFT value is the temperature at which the adhesive fails. Another standard thermal test involves a Static Shear Test done at a respective constant temperature, such as 80°C. The Shear Value reported would be the time required for adhesive failure at the respective elevated temperature.
Factors Influencing Properties
The properties of PSAs are influenced by certain factors. Each component in a formulation plays an important role in determining the end properties of that respective adhesive. Even slight alterations in a formulation can drastically affect the adhesive's performance.The tack property is affected mainly by the crosslink density, or functionality, of the adhesive. A lower crosslink density will generally result in a higher tack value. This will allow the adhesive greater flexibility and assist in its conforming to and wetting out upon substrates. Most radiation-curable PSAs consist of inert and monofunctional products with very limited exceptions of di- and tri-functional components. Another vital component of tack involves the glass-transition temperature (Tg) of the adhesive. As a general rule, the lower the overall Tg, the higher the tack value will be. A typical PSA will be have a Tg of between -25°C and +5°C, with the lower Tg products having increased tack performance. Another method of increasing the tack property is to add flexibilizers to the adhesive, such as benzoate plasticizers, which assist in the conformation of the adhesive.
The adhesion property is a balance between tack and shear performance. Low Tg products that have good cohesive properties exhibit high peel performance. These are typified by large-molecular-weight urethane acrylates with mono- or difunctionality and low Tgs between -10°C and -30°C. The larger molecular weight assists with the cohesion attributes of the adhesive. Low Tg monomers, like isodecyl acrylate, are also beneficial to enhanced peel properties rather than high Tg monomers, such as isobornyl acrylate. Finally, increasing the content of inert tackifying resins generally benefits the adhesion performance by maximizing the elasticity of the elastomeric component.
The shear performance of a PSA illustrates the cohesion of the system. The fundamental method of enhancing the shear property is to increase the crosslink density of the adhesive. This is accomplished by increasing the functionality of the system's components. In addition, an increase of the overall Tg of the adhesive will assist in achieving this goal. By using higher Tg tackifying resins, diluents or oligomers, the shear values of a PSA will rise significantly. These trends also hold true for the heat resistance property of an adhesive, resulting in increased SAFT values.
Specific Formulations
High-Tack UV-Curable PSAThe high tack of the formulation in Table 1 derives from the low functionality and Tg's of the products included. The GENOMER* 4188 is monofunctional, and, when combined with 2-EHA, it offers exceptional elastic properties that are ideal for PSA's. The GENOMER* 6043/M22, an inert resin cut in a monofunctional aliphatic urethane acrylate monomer, is being utilized as a low Tg (-18

UV-Curable PSA Formulation
To maximize the shear and temperature-resistance performance of a PSA, the objective is to increase the crosslink density and overall Tg of the adhesive. This is accomplished by replacing the inert tackifying resin with a difunctional aliphatic urethane acrylate, GENOMER* 4269/M22, and adding 4% of a trifunctional polyester acrylate, 03-849 (see Table 3). The increased crosslinking and Tg negatively affects the tack property, but greatly enhances the characteristics that are targeted. Using MIRAMER* M130 isodecyl acrylate as the diluent also works very well for higher temperature resistance.

The formulation in Table 4 offers nicely balanced PSA performance with exceptional adhesion and shear properties. This is done by reincorporating the tackifying resin (Co-Resin 02-819/M22) to allow the oligomeric component to maximize its elastic characteristics and to decrease the crosslink density, which act to increase the peel and tack properties while still maintaining excellent shear and heat resistance.
Conclusion
The pressure-sensitive adhesive starting point formulas serve as a basic reference tool for formulators. These formulations can be readily modified to specific applications. The concept of this study is to not only offer the formulator functional PSA formulations, but to provide an insight into the methods of altering certain properties that will enable a desired endpoint. It is evident that the delicate relationships between the oligomers, co-resins, different types of monomers and photoinitiators must be perfectly balanced in order to achieve a functional and optimized pressure-sensitive adhesive.For more information, contact RAHN USA Corp., 1005 North Commons Drive, Aurora IL 60504; phone (630) 851-4220; fax (630) 851-4863; e-mail EnergyCuring@rahn-group.com ; or visit http://www.rahn-group.com .
