
- Odorless sulfopolyester,
- Readily dispersible in water,
- Non-dispersible in ionic environments, and
- Good adhesive performance characteristics.
The purpose of this article is to introduce this new, odorless polyester as a raw material for formulating water-dispersible hot melt adhesives.
Hot melt adhesives are useful for bonding various substrates such as wood, paper, plastics, nonwoven assemblies, textiles and other materials. One use for which they are well suited is the sealing of corrugated and paperboard boxes. This application calls for high bond strength to resist shock, stress, high humidity and extreme temperatures encountered in transportation and storage. In addition, the melt point, wetting time, initial tack, setting time, pot life and general handling characteristics on automatic packaging machinery are essential considerations.
Paper products must be recycled to conserve material resources and landfill space. It is thus a general practice in the paper industry to recover the used and waste material and to repulp it for use in products such as cardboard. The use of polyolefin-based hot melt adhesives to seal boxes has presented problems in repulping. Hot melt and pressure sensitive adhesives are generally insoluble in water and very difficult to disperse during the repulping process. In addition, many polymer emulsions are non-redispersible. This makes certain paper products that utilize insoluble adhesives unattractive for repulping since the adhesive particles can introduce inconsistencies into the finished product.
One way to remove insoluble adhesives from waste paper products is to use adhesives having a density different from that of water and pulp in water, thus permitting gravitational separation. However, this requires separation steps that can increase recycling costs. In addition to paper and paper products, there are other disposable items, such as diapers and sanitary napkins, in which hot melt and aqueous adhesives are used. However, the use of most hot melts in these products complicates the disposal process. Accordingly, it would be most desirable to produce a relatively low-odor, water-dispersible hot melt adhesive that maintains the desirable properties of non-dispersible adhesives.
Technical Aspects of Polyesters
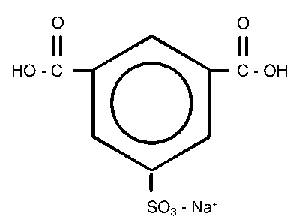
Conventional water-dispersible polyesters are linear, amorphous materials composed of aromatic difunctional acids and aliphatic glycols, and range in glass transition temperature (Tg) from 29°C to 55°C. Their water dispersibility is due to the presence of pendent sodiosulfo groups randomly distributed along the polymer backbone. Incorporation of the ionic moieties is readily accomplished by copolymerization of 5-sodiosulfoisophthalate units into the polymer backbone. (See Figure 1.)While the linear sulfopolyesters are very useful in many applications including adhesives, their use in hot melt adhesives is limited due to their Tg and melt viscosities being relatively high. To address these concerns, Eastman developed a family of sulfopolyesters with branching in the polyester backbone. The lower Tg of the branched sulfopolyesters not only results in better adhesive performance but also improves the water dispersibility by enabling the dispersion to occur at lower temperatures. These improvements have led to use in a variety of hot melt adhesive applications.
Although adhesive performance is improved, the branched sulfopolyesters can have a distinct odor that is not desirable in selected applications. The new odorless sulfopolyester represents the advantages of both the linear and branched compositional families.
Eastman's conventional linear sulfopolyesters are inherently low-odor based on their combination of acids and glycols, while the branched sulfopolyester line has lower aromaticity and a level of branching that results in a low melt viscosity with retention of tensile/adhesive properties. The odorless sulfopolyester has a narrower molecular weight distribution (MWD), but retains the desired performance attributes via structural modification. This structural modification results in a sulfopolyester that possesses a very high degree of hydrophilicity to allow for additional formulation latitude.
A higher level of aromatic linkages also provides increased hydrolytic stability for dispersion-grade products. High-clarity dispersions at concentrations up to 30 weight percent are obtained with the odorless composition at low viscosities. Adhesive performance is comparable to the branched sulfopolyester with a similar viscosity.
Competitive Matrix
The odorless sulfopolyesters along with the branched sulfopolyesters are designed to replace hydrophobic products, such as ethylene-vinyl acetate (EVA) copolymer and styrene/isoprene block copolymer. Shown in Table 1 is a comparison of currently employed technologies demonstrating enhanced adhesion with sulfopolyesters.Product Function
The products are inherently water-dispersible due to the random incorporation of 5-sodiosulfoisophthalate units within the polyester backbone. The compatibility with a range of commonly employed hydrophobic raw materials, such as acid functional and/or aromatic- or cyclic-structure tackifying resins, glycol-containing oils, or cycloaliphatic plasticizer, allows for a variety of hot melt adhesives to be formulated. These finished adhesives are then rendered water-dispersible by the efficacy of the polyester as a surfactant. The recyclability or flushability of nonwoven, adhesively bonded articles is enhanced; for example, a disposable diaper in a waste-treatment environment process is not affected by the presence of insoluble masses of adhesive.The uniqueness of the polyester stems from its tailored molecular architecture. A proprietary process has allowed a structure to be manufactured within narrow ranges of melt viscosities. This results in a broad MWD where there are enough low-molecular-weight fractions to provide the low melt viscosity, while at the other end of the spectrum there are sufficient numbers of high-molecular-weight species to give the tensile and adhesive properties that are needed.
Another key breakthrough was the discovery of a monomer composition that did not just lower the Tg and improve adhesive performance but also provided a high ring-and-ball softening point (RBSP). Finally, the same ionic nature of this polyester that results in water-dispersibility also prevents solubility in ion-containing body fluids.
Product Application
The amorphous sulfopolyesters are thermoplastic rather than thermosetting, and they can therefore be re-melted to form a heat-sealable bond. Sulfopolyesters offer a good combination of properties when used as base polymers in hot melt or aqueous dispersions for paper products. They are easily dispersed and recycled. They offer significant improvements over current dispersible hot melt adhesive raw materials in strength and thermal stability. Furthermore, they offer improved adhesion, especially with respect to treated polypropylene and polyethylene.A variety of disposable products, such as diapers, sanitary napkins and incontinent briefs would be more environmentally friendly if a water-dispersible hot melt adhesive could be used. For example, a branched sulfopolyester quickly dissolves into a compost pile; this would facilitate the breakup of a diaper assembly and hasten the degradation by virtue of the more rapid increase in surface area.
Another example could be the design of a flushable sanitary napkin. Once again, the assembly would undergo fragmentation more rapidly, which, in turn, would hasten degradation. It would be an understatement to say that the non-dispersibility of sulfopolyester in body fluids (urine, sweat) is an essential innovation characteristic to spur these types of product developments.
The sodiosulfo groups introduce charged functionalities that electrostatically stabilize the polymer molecule in pure water. The remainder of the sulfopolyester, although relatively polar, is not inherently water-soluble. Because the ionic groups are randomly distributed along the backbone, it is unlikely that a classical micelle structure is formed where all the charged groups reside on the surface of a particle with the hydrophobic segments occupying the interior.
However, sulfopolyesters are not known to form true solutions, but rather exist as dispersions of particles in water. Therefore, it is reasonable to expect that the surface of the particles would contain a greater proportion of the ionic groups to provide water dispersibility.
The discrete particle phase is a key to the non-dispersibility in saline solutions, because adding salt to the water in essence causes the water to become a poorer solvent for the sulfopolyester. Adding ionic species to the water will invoke a situation of electrostatic repulsion where the polymer particles will have no driving force to form a dispersion.
Viewing this situation from the opposite perspective where the polymer would be already dispersed and salt then added to the aqueous medium, it would be possible to precipitate out the polymer. Thus, ionic strength may be used as a tunable solubility mechanism for recovery of the dispersed polymer. Increasing the ionic strength of the aqueous medium to a high enough level will cause the polymer particles to overcome their repulsion, coagulate and form a separate phase.
Physical Properties of Odorless Sulfopolyesters
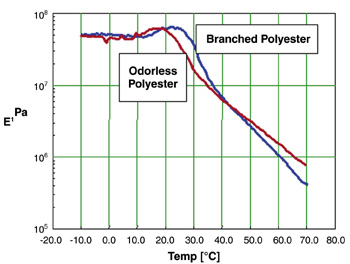
The Tg of the polyester is about 11°C, as measured by Differential Scanning Calorimetry (DSC). Brookfield Thermosel viscosity's run on a Model DV-1+ viscometer indicates that the polyester has a viscosity at 177°C of 39,000 cP. The odorless polyester has an acid number of 4 and a hydroxyl number of 23.To evaluate the rheology properties of this new polyester, storage modulus and dynamic mechanical analyses were performed using a RMS-800 rheometer.
A comparison between the standard branched sulfopolyester and the new polymer is in Figure 2.
Results of the analysis indicate similar molecular storage integrity. The instrument in the analysis is a Rheometrics solids analyzer (RSA II). The temperature ramp for the samples was started at -50°C and increased 5°C per minute until a temperature was reached where the E' began to drop off sharply (70°C) and most of the sample had been pressed out of the plates. The data from -50°C to -10°C were cut because the E' was essentially a straight line until it reached -10°C.
Incorporation Into Adhesive Formulations
The unique polyester can be combined with a wide range of other commonly used adhesive raw materials. The tackifying resins useful in adhesive compositions are generally polar in nature and have a RBSP greater than 80°C. Water dispersibility and compatibility with a variety of tackifying resins/rosins were evaluated. Results indicate excellent compatibility with resins/rosins with aromatic, cycloaliphatic or highly acid-functional chemical structures.Various plasticizing or extending oils may be incorporated into the base polyester. Compatible plasticizers include white mineral oils and benzoate plasticizers, such as dipropylene or diethylene glycol dibenzoate and 1,4-cyclohexane dimethanol dibenzoate.
Among the applicable stabilizers or antioxidants that may be used are high-molecular-weight hindered phenols and secondary antioxidants containing sulfur or phosphorus. The odorless sulfopolyester is manufactured with both primary and secondary antioxidants.
Statistical Summary of Adhesive Performance
Objective: To examine changes in various adhesive characteristics by varying the formulation by incorporation of an Optimal Mixture Design capable of capturing interaction effects.Factors:
- Polymer, ranging from 50% to 100% of the formulation;
- Plasticizer (oil), ranging from 0% to 30% of the formulation;
- Tackifier (resin), ranging from 0% to 50% of the formulation
Responses:
- Viscosity @ 162°C
- Viscosity @ 177°C
- RBSP (°C)
- Polyken Tack (g/cm2)
- Loop Tack (g/mm)
- 180 Peel (g/mm)
- T Peel (g/mm)
- 50°C Hold Power (hours)
- RT Hold Power (hours)
- SAFT (°C)

Boundary points denote the experimental region of polymer from 50% to 100%, oil from 0% to 30% and resin from 0% to 50%.
Data Analysis
A model was fit to each response. The two types of models fit to this data set are the linear model and the quadratic model. The linear model was fit if there was no evidence of significant interactions. That is, the polymer, oil and resin blended linearly. The quadratic model was fit if there was evidence of significant interactions. That is, the polymer, oil and resin blended nonlinearly. RBSP was the only response that could be modeled by simply using the linear model. All the other responses required the quadratic model.The formulations incorporated in the mixture design are in Table 2, and contour plots with statistical analysis for each plot are in Figures 4-8. Based on the overall data, formulation Number 4 in Table 2 provides tack and peel values suitable for hot melt nonwoven adhesive applications.
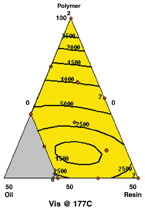
At both temperatures, the model includes interactions of polymer/plasticizer and polymer/tackifier. The viscosities increase as the formulation approaches 100% polymer.
The boundary points in Figure 3 denote the experimental region of polymer from 50% to 100%, oil of 0% to 30% and resin from 0% to 50%. Each contour plot also shows boundary conditions.
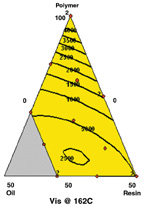
At both temperatures, the model includes interactions of polymer/plasticizer and polymer/tackifier. The viscosities increase as the formulation approaches 100% polymer.
Contour Plots With Statistical Analysis
Viscosity at 177°C and 162°C — At both temperatures, the model includes interactions of polymer/plasticizer and polymer/tackifier (figure 4).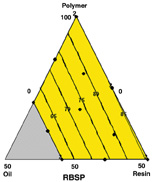
None of the RBSP interactions are significant. The plot shows that the RBSP increases as the formulation approaches 0% plasticizer

The model does include the interactions of polymer/plasticizer and plasticizer/tackifier. The plot shows that the loop tack increases as the formulation approaches approximately 15% plasticizer, 85% polymer.
Polyken tack — The Polyken tack is maximized as the formulation approaches approximately 25% plasticizer regardless of the levels of polymer and tackifier.
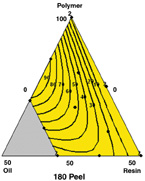
The model includes the interactions of polymer/plasticizer and plasticizer/tackifier. The plot shows that 180 peel increases as the formulation approaches 25% plasticizer, 75% polymer.
180 Peel —The model includes the interactions of polymer/plasticizer and plasticizer/tackifier. Figure 8 shows that 180 peel increases as the formulation approaches 25% plasticizer, 75% polymer.
T Peel — The model includes the interactions of polymer/plasticizer and plasticizer/tackifier. T Peel increases as the formulation approaches approximately 15% plasticizer, 35% tackifier and 50% polymer.
50°C Hold Power — It includes all the two-way interactions, which are polymer/plasticizer, polymer/tackifier and plasticizer/tackifier. As the formulation approaches 50% tackifier and 50% polymer, the 50°C Hold Power increases.
RT Hold Power — The model includes the interactions of polymer/plasticizer and plasticizer/tackifier. RT Hold Power increases as plasticizer approaches 0% regardless of polymer and tackifier.
SAFT — The model includes only one interaction, plasticizer/tackifier. The SAFT increases as the plasticizer approaches 0%.
Results and Conclusions
The results of this study indicate that the odorless, water-dispersible polyester should find considerable utility in a wide variety of recyclable hot melt adhesive applications. With the aid of the contour plots, water-dispersible hot melt adhesive formulations can be identified for nonwoven assemblies and pressure sensitive applications. In each of these areas, the odorless, water-dispersible sulfopolyester provides for a unique combination of performance properties, including excellent adhesion to polyethylene, a wide latitude of adhesive performance, and water dispersibility in neutral or alkaline conditions, yet non-dispersibility in ionic solutions such as body fluids.This article is based on a presentation made at the 2000 TAPPI Hot Melt Symposium, Bal Harbour, Fla. Additional information about odorless, water-dispersible sulfopolyester is available from Eastman Chemical Co., Lincoln St., Kingsport TN 37662; phone: 423-229-2000; fax: 423-229-1064; e-mail: ramiller@eastman.com; Web site: www.eastman.com.