The utilization of Attenuated Total Reflectance – Fourier Transform Infrared (ATR-FTIR) spectroscopy for the characterization of pressure-sensitive products is well established. Some recent advances in sensor technology have opened new application areas of ATR-FTIR spectroscopy. One of them is the monitoring of chemical reactions, which are of interest for pressure-sensitive adhesive production. In this study, ATR-FTIR spectroscopy was used for monitoring polymerizations of acrylic monomers. Current results offer a basis for the development of ATR-FTIR spectroscopy as not only a monitoring tool, but also as a tool for process control.

(Published with permission from Ref. 5)

(Published with permission from Ref. 5)
In this study, a commercially available ATR-FTIR reaction monitoring system was used. It operates in the MID IR region (4000-650 cm-1) and consists of electronic and optical modules. A purged path for the IR beam from source to detector and back was ensured using a set of mirrors and conduits ending with a remote sampling device. The sampling device consists of a stainless steel body and a six-reflection bi-layer diamond-composite ATR element. The external processor is used for data acquisition and manipulation. Details of the probe are discussed elsewhere.5 The basis for monitoring the reaction is the determination of characteristic absorbances assigned to monomer consumption or polymer buildup. These can be used to calculate polymer composition and overall conversion.
(Published with permission from Ref. 5)
Experimental Methods
Solution Polymerizations. Six homo- and co-polymerizations of butyl acrylate (BA) and vinyl acetate (VAc) in toluene were performed. The concentration of toluene was varied from 50 to 80 wt. percent. N-dodecyl mercaptan was used as a chain transfer agent and 2,2’- azobisisobutyronitrile (AIBN) as an initiator. Standard procedures were followed for reagent purification.5All reactions were performed in glass ampoules at 60 degrees C. For each measurement, the reaction in two ampoules was simultaneously quenched and the contents of one ampoule were analyzed by gravimetry and1H-NMR (Proton Nuclear Magnetic Resonance) spectroscopy. The contents of the second ampoule were used for off-line determination of conversion and copolymer composition using the ATR-FTIR probe. The probe was inserted into a vial containing the reaction mixture. For all samples, air was collected as a background spectrum and automatically subtracted from the sample spectra. All spectra were collected at 64 scans and a resolution of 8 cm-1.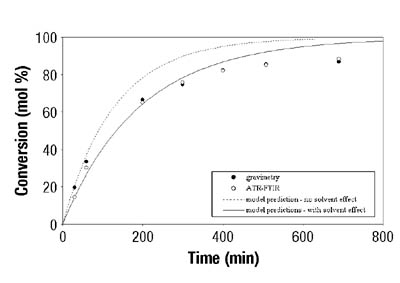
(Published with permission from Ref. 5)
(Published with permission from Ref. 5)
Results and Discussion
Solution Polymerization Monitoring. In this part of the project, the main objective was the identification of characteristic absorbances and their utilization for copolymer composition and overall conversion monitoring. Given this objective and the nature of the experimental runs, off-line measurements were a good initial start for the development of a measurement method based on ATR-FTIR spectroscopy. The results obtained were compared to gravimetric measurements.
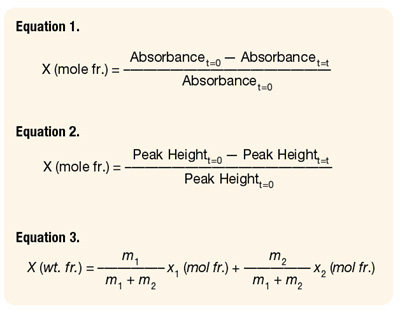
The basis for the application of ATR-FTIR spectroscopy in monomer conversion determination is Beer’s law. The absorbance of the component in the reaction mixture is directly proportional to its concentration. The absorbance can be measured as peak height, peak height ratio, peak area or peak area ratio. Thus, a simple expression for conversion is obtained (Equation 1):
(Published with permission from Ref. 5)
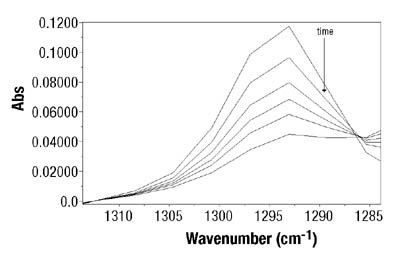
After peak identification, the next step was the determination of the best mathematical expression for the absorbance. Theoretically, regardless of the peak selection and the expression for absorbance, the results should be similar. However, in this case, the best fit between gravimetry and ATR-FTIR data was obtained when the absorbances at 810cm-1for BA and at 1293 cm-1for VAc (see Figure 2) were used. A peak height to two-point baseline was used in both cases. Thus, Equation 1 becomes (seeEquation 2):
Figures 3-4 show typical results for BA and VAc homopolymerizations in toluene. Similar results were obtained when different solvent concentrations were used. In all cases, ATR-FTIR spectroscopy showed good agreement with the standard gravimetric method. The estimated error for ATR-FTIR spectroscopic data was 2.5 wt. percent.7
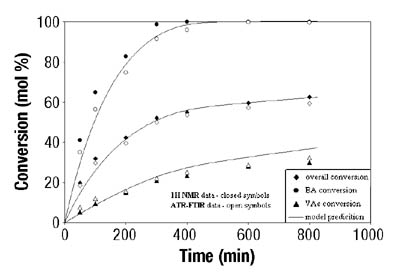
After the determination of conversion vs. time data of the solution homopolymerizations, ATR-FTIR spectroscopy was used for off-line monitoring of BA/VAc solution copolymerizations. In addition to low toluene absorbance, an additional challenge was to find the characteristic absorbances for each of the two monomers that would not overlap, in order to use them for the determination of individual monomer conversions. Figure 5 shows the results are shown for a BA/VAc solution copolymerization in toluene.
In the copolymerization case, the following expression was used to calculate overall conversion using ATR-FTIR spectroscopic data (see Equation 3):

(Published with permission from Ref. 6)
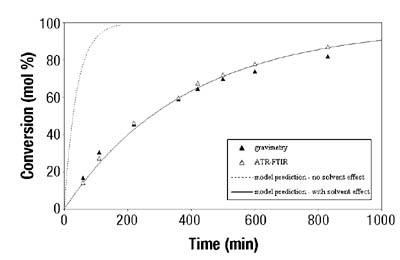
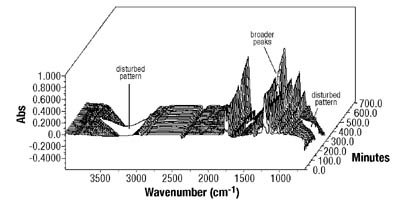
Emulsion Polymerization Monitoring. After the successful completion of off-line monitoring of homogenous reaction systems, the ATR-FTIR probe was used for the more complex, in-line monitoring of the heterogeneous emulsion polymerization system. Real-time monitoring of such a system is interesting not only because there are several phases present, but also because of the compartmentalization of monomer(s) among them, especially for highly water soluble monomers such as VAc. Typical reaction spectra collected in-line are shown in Figure 6. Real-time peak profiles, i.e., normalized absorbances vs. time (Figure 7) were used as input data in Equation 2 to determine the individual monomer conversions. Once the individual monomer conversions were obtained, Equation 3 was used to determine overall conversion.

(Published with permission from Ref. 6)

(Published with permission from Ref. 6)

(Published with permission from Ref. 6)
(Published with permission from Ref. 6)
Conclusion
For the production of PSA with the desired built-in performance characteristics, real-time process monitoring and control are essential process tools, especially for systems where compositional drift can occur or where variation in physical and chemical properties of reactants is expected. Even though there are different techniques for reaction monitoring, ATR-FTIR spectroscopy has advantages over other monitoring techniques. It has a potential for real-time monitoring of kinetic and structural changes of the reaction components with no requirements for sampling loops or complex changes to reactor design.
In this study, it has been shown that an ATR-FTIR probe can be successfully used for off-line and in-line monitoring of homogeneous and heterogeneous polymerization systems that are commonly employed in PSA synthesis. Used in the study for off-line monitoring of BA/VAc solution polymerizations, it has accurately monitored conversion and copolymer composition. When used off-line, no disadvantages were observed. The time lag was minimal, no calibration was required and the technique was accurate compared to standard techniques. When similar principles were applied for in-line monitoring of BA/VAc emulsion polymerizations, in most cases the probe was able to correctly monitor copolymer composition and conversion compared to traditional techniques. When used in real-time, the probe was also able to follow the occurrence of an induction period or to offer an early indication of catastrophic coagulation. On the other hand, strict temperature control was found to be a major requirement for the successful application of ATR-FTIR spectroscopy. The extent and the duration of the disturbance were major factors affecting the performance of the probe. In addition, some problems were experienced at high concentrations of water-soluble monomer combined with PVOH presence and high solids content. At this point, one speculation is that the methods developed for other feed compositions might not be applicable when grafting reactions are likely. The performance evaluation of the probe for real-time monitoring of miniemulsion polymerizations is still in progress.
Acknowledgements
The authors wish to acknowledge the National Engineering and Science Research Council of Canada and the Canada Foundation for Innovation for the financial support of this project.References:
1 Benedek, I.; Heymans, L.J. Pressure-Sensitive Adhesives Technology, Marcel Dekker Inc.: New York, 19972 Dallin, P. Proc. Control Qual. 1997, 9(4), 167-172.
3 Hergeth, W.D. In Polymeric Dispersions: Principles and Applications. Asua, J.M. Eds.; Kluwer Academic Publishers: Dordrecht, 1997; 267-288.
4 Kammona, O.; Chatzi, E.G.; Kiparissides, C. J. Macromol. Sci.—Rev. Macromol. Chem. Phys. 1999, C39(1), 57-134.
5 Jovanovic, R.; Dube. M.A. J. Appl. Polym. Sci. 2001, 82(12), 2958-2977.
6 Jovanovic, R.; Dube. M.A. Polym. React. Eng. J. (in press).
7 Hua, H.; Dube. M..A. J. Polym. Sci.: Polym. Chem. 2001, 39, 1860-1876.