
Despite the proven performance advantages of ultraviolet (UV)-cure pressure sensitive adhesives (PSAs), formulators have often been forced to seek alternative solutions due to the limited range of raw materials available and comparatively high prices. Many formulators opted instead for more economical cure methods, such as solvent-based, water-based and hot melt technologies.
But today, more chemists and formulators are turning to UV-cure technologies. In fact, a post-9/11 report by the Freedonia Group, Cleveland, predicts that radiation-cure adhesives will explode at a rate of 10% per year, reaching almost eight million pounds in 2005. The report also predicts that the fastest growth will occur in PSAs and other small-volume adhesives because of a gradual reduction in raw material costs.
EPA regulations have also helped ensure a healthy future for UV-cure PSAs. Recent regulations force manufacturers across a broad range of industries to switch to no- or low-VOC technologies.
An Introduction to UV-Cure PSAs
Three main properties determine if a product can be labeled a pressure sensitive adhesive - "tack," "peel" and "creep." Tack is the property related to bond formation. Peel is the tensional force required to remove adhesive tape. Finally, creep measures the flow characteristics of the PSA.Because these properties are critical to the adhesive's performance, formulators must be aware of any factors that can directly affect them - such as temperature, aging, film thickness, cure rate and post-cure parameters. They must also be aware of formulation variables - including oligomer selection, tackifier addition, monomer structure, molecular weight and glass transition - that directly impact tack, peel and creep.
Traditionally, PSAs have been developed around rubber-based formulations with the inclusion of rosin esters or C-5 and C-9 hydrocarbon resins as "tackifiers" - those resins that promote the "sticky" feeling of PSAs.
Advantages of UV-Cure Technology
UV-cure PSAs offer several advantages over conventional or solventborne PSAs. Recent growth in the UV PSA market can be attributed to three UV-curePSA benefits:
-
1. Improved performance properties,
2. Simpler environmental compliance, and
3. Reduced prices.
Improved Performance Properties
As the test results in this article will indicate, UV-curable PSAs can be formulated and processed to deliver a broad range of performance properties, making them a viable option for many industrial applications. Of particular interest are specialty applications demanding high-temperature stability and solvent resistance. Choice of tackifier resin, monomer and oligomer is critical in determining these performance criteria.Simpler Environmental Compliance
Solventborne PSA formulations traditionally based on modified rubber chemistry are now being challenged by government mandates requiring low-VOC standards. UV-cure PSAs and other environmentally sound technologies enable formulators to meet these regulations.For example, UV-curing waste can be disposed of as ordinary solid waste, increasing environmental safety and offering significant savings. Additionally, UV PSAs are generally safer for formulators to handle than the solvent materials they replace, offering lower skin and eye irritation.
Environmental and safety benefits, coupled with the desire for fast-curing UV/EB systems, are creating a fast growth market for UV PSAs.
Reduced Prices
Formulators are beginning to realize that in the long run UV PSAs may offer a better value and are willing to pay for the performance they deliver. UV curing decreases the drying time required to complete a process, which reduces the overall run time for a variety of applications. This, in turn, increases production capacity and throughput rates, requires less direct labor, and decreases downtime. In addition, UV-cure systems cost approximately 50% less than large-capacity thermal systems.
On top of these benefits, raw materials prices for UV-cure PSAs are gradually declining - and are expected to continue doing so in the next few years.
Manipulating UV-Cure PSA Performance
This study - involving 12 monomers and three tackifiers at varying concentrations - provides insight into the trends that influence the quality of PSA performance properties. It examines individual properties and explains their influence on specific applications.
Formulation:
Eleven monomers, three tackifiers and CN-966 (a highly flexible urethane diacrylate) were blended at 40 deg C. Homogeneous mixtures were catalyzed at 25 deg C to achieve formulation stability. The oligomer and monomers formulated contained commercial stabilizer levels, and the purity was that of commercially available products. Molecular weight and chemical structure were two factors also considered in this study. (See Table 1.)Monomer Selection:
The selected monomer blend included ethoxylated, trifunctional and monofunctional acrylates incorporated at 40 percent into the base formulation. The acrylate monomers provided adhesives with different crosslink densities, solubilities, glass-transition temperatures and flow characteristics. Fifty percent CN-966, the highly flexible urethane acrylate, and 10% photoinitiator made up the balance of the formulation.
Tackifiers:
In this study, three tackifiers were formulated into PSAs. Solubility, viscosity, tack, creep and peel - properties required in adhesives - were tested. Table 2 provides the melt points of a pentaerythritol-based rosin ester, C5/C9 resin and C9 resins when tested in a variety of oligomer and monomer formulations.Oligomer Selection:
High-molecular-weight and flexible polymers provided PSA stress distribution. CN-966 provided a crosslinked segment, or block, within the cured PSA. Energy transmitted to the polymer or oligomer was distributed across the adhesive's surface area. CN-966 provided the aliphatic polyester isocyanate backbone that yielded excellent weathering resistance and stability from post-aging degradation.Photoinitiator:
In this study, benzophenone (4%), methyldiethanolamine (3%) and Esacure KIP100F (3%) photoinitiators were selected, optimizing cure efficiency. KIP100F provided excellent bulk-cure efficiency, increasing the molecular-weight build. Benzophenone and methyldiethanolamine ensured that the surface tack exhibited by the cured adhesive was not attributed to uncured monomer at the surface of the PSA.Application and Cure Parameters
Application: PSAs were applied to Mylar films. Wire-wound drawdown bars applied the adhesive at 0.0005 to 0.006 inches to test properties vs. adhesive thickness.
Cure Parameters:
Adhesives were cured using a 300 watt/inch, medium-pressure mercury-vapor lamp at 4, 8 and 16 meters per minute. Tack was measured vs. cure rate to enhance post-age performance. The outcome proved that monomer selection was directly related to cure rate vs. adhesive properties. Eight out of 11 monomers lost tack with increasing total cure energy.Study Results
Triacrylates: Rapidly increasing molecular weight and crosslink density causes the product to become tack-free, hard and solvent-resistant. These properties make triacrylates suitable for coatings. However, the high crosslink density makes high concentrations unsuitable for use in PSAs. Increasing the molecular weight and lowering the glass-transition temperature improve the rolling-ball tack at fast cure speeds. (See Table 3.) Rolling-ball tack decreases as product crosslink density increases, again, making triacrylates unsuitable for PSAs.
Photoinitiator selection was designed to accelerate post-cure phenomenon characteristics. As stated earlier, the property-forming bond is called tack and the tensional force required to remove the adhesive tape is called peel.
Again, the third property is flow resistance or creep. A broader sense would include the PSA's entire stress/strain behavior. Rolling-ball tack was measured using a 0.8-cm stainless steel ball rolled from a 6-cm height at a 20 deg elevation. The distance the ball rolls from the incline base to the point when forward motion is stopped was measured and recorded. Remember that rolling ball tack (RBT) does not measure adhesive bond strength. Rather, it measures adhesive flow and wetting characteristics.
When dealing with monomer structure, molecular weight significantly affects rolling-ball tack. Urethane acrylate concentration, tackifier and adhesive formulation thickness determine PSA properties.
Four monofunctional acrylates were tested extensively. The acrylates ranged from highly polar to non-polar, and lowest molecular weight of 188 to highest molecular weight of 450. Polyethylene glycol, pure alkane and aromatic-ring chemistry directly influenced PSA performance.
Monomer Structure:
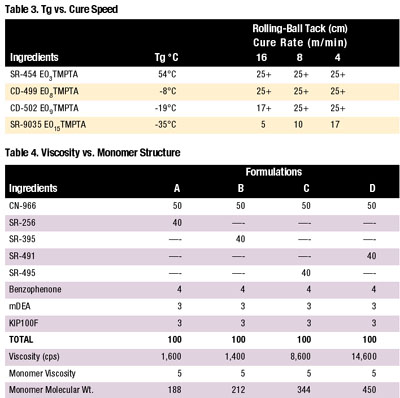
Viscosity measures the freedom of movement the formulation ingredients possess in the liquid state. SR-395, or isodecyl acrylate (IDA), which is hydrophobic and non-polar in nature, provided excellent solvency characteristics. The CN-966 coupled with SR-395 (five-cps viscosity) yielded consistently lower formulation viscosity. SR-256 or ethoxy-ethoxyethyl acrylate (EOEOEA), which is a high polarity monomer, yielded viscosities from 10% to 15% higher than SR-395. SR-256 has a viscosity similar to SR-395 in the five-cps range. All of these monomers are excellent diluents.
As shown in Table 4 (page 36), SR-495 or monohydroxy caprolactone acrylate yielded significantly higher viscosities. Molecular weight increase accounted for the change. The large monomer molecular weight resulted in less freedom of movement for all formulation ingredients, thereby increasing viscosity.
Cure Rate:
Cure rate influences rolling-ball tack through monomer structure. SR-256 provided excellent cure speed due to its high crosslink density and an ethoxylated, alpha-hydrogen-rich backbone. Isodecyl acrylate, SR-395, cured the slowest with no alpha hydrogens. SR-495 cured slightly faster than SR-395 due to the adhesive's ability to increase molecular weight faster.SR-395 cured the slowest, enhancing the cure rate vs. rolling-ball tack. RBT decreases when monomer level increases in the formulation. This is true for all the monofunctional monomers tested.
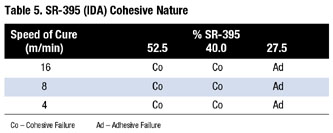
However, SR 395-based formulations decrease in RBT with increasing energy. SR-395's slow curing results in molecular weight increase with increased energy. Table 5 reveals the cohesive nature with large volumes of SR-395 or small energy applied to the adhesive.
The SR 395-based adhesive split leaves residue on both the test surface and Mylar film. It is important to note that a PSA should have adhesive failure. SR-256 and SR-495 failed adhesively to metal surfaces tested.
Monoacrylate molecular weight itself affects RBT to a lesser degree. Increasing monomer molecular weight from SR-256 (molecular weight 188) through SR-395 (molecular weight 212) then up to SR-495 (molecular weight 344) showed a slight improvement in RBT using tackifier resins. Increasing monoacrylate molecular weight showed a trend when increasing RBT up to that of SR-495. Lower values indicate better RBT. The non-polar nonyl carbons and phenyl rings produced the hard segment. This hardness resulted in less RBT.
Acrylate homopolymers resulted in a highly polar, flexible block. The non-polar nonyl carbons and phenyl rings produced the hard segment. This hardness also resulted in less RBT.
Film thickness is critical when testing UV-cured PSAs. Film thickness applied from 0.0001 to 0.003 inches (25 to 75 microns) minimally affected PSA properties. However, there was a notable decrease in RBT with increasing adhesive-film thickness.
Peel:
Peel adhesion was measured by using a one-inch adhesive strip applied to a polished stainless steel plate. The adhesive was applied using a 2,770-gram roller as the force to set the bond. The weight was rolled over the adhesive twice to ensure uniform testing for all materials.
The adhesive tape was then peeled at a 180 deg angle at one-inch-per-second (2.54 cm/sec) peak peel with average peel data recorded. Peel strength depends on cure rate, monomer molecular weight/structure, tackifier resin and oligomer level.
Peak Peel:
Peak peel strength represents the break force, or energy, required to initiate PSA failure. SR-395 dramatically changed rolling-ball tack vs. cure rate. The peel adhesion test did not have the same large change. However, with the increased energy level, the crosslinking of SR-395 did increase the peak peel values.When increasing SR-395 [or decreasing cure rate to 16 m/min (50 ft/min)], cohesive failure resulted. In addition, SR-395 odor remained in the finished adhesives.
Monoacrylate molecular weight continued to play an active role in peak peel strength. SR-256 is a highly polar monomer particularly suited to Norsolene S-95 (C9 hydrocarbon resin).
The C9/SR-256 PSA yielded 400+ gm/inch peel strength. This is similar to desk tape with 400 gm/inch peel strength. The SR-256 adhesives similarly had rolling-ball tack and creep resistance with the same performance.
The non-polar alkane structure, SR-395, yielded exceptional peel strength formulated with the non-polar CN-966, or the pentaerythritol-based rosin ester. It was noted that
S-95, a C9 resin, did not increase the physical properties. A proposed explanation is the difference in structure and polarity. The SR-395 did exhibit residual odor despite the excellent peel strength.
The caprolactone acrylate did not show good compatibility with any tackifier. The hydroxy group provided polarity, and the caprolactone did not offer solvency characteristics. The SR-495 would provide good weathering and a hydroxy group for dual-cure mechanisms. Reacting SR-495 with an anhydride, or isocyanate, through post-cure would make a good UV-curable PSA, forming laminating-adhesive bonds.
Highly flexible, urethane diacrylate CN-966 did not significantly affect the peak peel strength. It was found that SR-395 did go through an optimization point at 50%. The high peel strength (1,240 gm/cm) did not enhance the creep resistance. The SR-395 creep resistance did improve with increased urethane acrylate.
SR-395's peak peel increased by adding 12.5% tackifiers. Increasing tackifier to 25% did not further enhance properties. It was found that increasing CN-966 urethane diacrylate significantly increases peak peel strength over the tackifier additions.
Rolling-ball tack and peak peel strength depend on monomer structure/molecular weight, concentrations, tackifiers, cure, and adhesive thickness. Additional perspective examines rolling-ball tack vs. peak peel strength.
Testing found that high rolling-ball tack (low distance in cm) yielded lower peak peel strength. Tack decreased (increase in RBT distance in cm) while peak peel strength increased. The four data points are noted as the four monofunctional monomers tested. S-95 increased rapidly, peaking in the SR-395.
Peak peel was very dramatic. The data is the same as was found above, however, making the monofunctional monomer the important variable.
The SR-495 or caprolactone acrylate consistently yielded high RBT and moderate peak peel strength. SR-256 or ethoxy-ethoxyethyl acrylate had high RBT and good peel strength. The performance properties of SR-256-based adhesives are similar to desk tape. Performance properties excelled that of high peel strength, high tack and cellophane packaging tape.

Creep:
Creep failure was only found for SR-395 formulations. The data in Table 6 show that cure rate, monomer structure and tackifier/oligomer concentration affect creep resistance.The 52.5% SR 395-based formulation developed little cystallinity, or creep resistance, despite the high cure energy. Note the steady improvement with increasing CN-966 urethane diacrylate content and speed.
The SR-495, and SR-256 passed the eight-day creep-resistance test. Incorporating 12.5% S-95 tackifying resin dramatically improved creep resistance based on SR-395 PSA. The tackifier resins increased the glass transition of the -36 deg C SR-395 monofunctional monomer.
Conclusions
The low-odor PSAs in this study can aid formulators in developing high-value, UV/EB-cured PSAs. CN-966 - a highly flexible urethane diacrylate - provides excellent peel strength and creep resistance when only used with monofunctional acrylate. It provides clear, compatible, and easily manufactured and stored PSAs. CN-966 also makes a good base for a wide range of additional tackifiers. Along with the pentaerythritol rosin ester, AR-95 and A-100 show compatibility with up to 25% tackifier concentration.Moving Forward
The field of UV-curable PSAs is one that will continue to rapidly grow due to their ease of handling, the latitude they provide in formulating and the environmental acceptability of these solvent-free systems. The nearly instantaneous cure reaction also enables extremely high-speed processing for cost efficiency. UV curing is also recommended for processing product substrates that cannot tolerate heat or that could be injured by high-energy radiation. Potential long-range applications of UV-cure PSAs include:- Tag and label manufacturing,
- High-temperature adhesives,
- Construction adhesives, and
- Packaging adhesives.
