
Dispersions of pigments containing different ions have been studied in waterborne latices in which the polymer contains ionizable functional groups. Each system has been tested for related adhesion properties when coated on metallic substrates. Vibrational spectroscopy has been used to analyze the polymer structural changes due to the presence of the different ions in these systems. This information has been extremely useful in elucidation of the conformational changes of the polymer associated with the ion-polymer interactions.
The polymer structural changes detected by infrared analysis have also been correlated with the overall adhesion and cohesion strength in the system. These results could have a significant bearing on the mechanism of the pigment-polymer association in waterborne coatings and adhesives. The results are discussed in light of the polymer charge density and two-phase condensation model for polyelectrolytes.
Call for Waterborne Adhesives
Specific factors, originating in both the public and private sectors, have created the need for replacement of some solventborne adhesive and coating systems with safer aqueous products. These include a number of EPA and OSHA regulations, the increased cost of solvents along with their dependence on petroleum, expensive hazardous waste disposal issues, and the danger of fire and related insurance risks. The environmental advantages of solvent-free products, therefore, have motivated their continued development and commercial applications.In the early development years of waterborne coatings, the products found application in very specific areas and were not able to replace solventborne coatings and adhesives since they all had serious performance problems. This was in part due to the lack of understanding of the chemical and behavioral characteristics of polymeric systems in aqueous solutions, which are far different from solvent systems.
Aqueous dispersion of acrylics, SBR, neoprene, vinyls (PVC), nitriles and natural rubber latices all have been used. In the early 1970s, aqueous urethane dispersions were introduced without much success due to the high cost and poor performance.
Recent technology advancements have resulted in dramatic improvements in waterborne resin dispersions, both from a chemical structure and an economic point of view. However, when these resins are used in formulation, their behavior and structural conformation do not stay the same and are subject to great changes. This is due to the interaction of the resin with its surrounding media.
Therefore, a basic understanding of the dynamic behavior of component molecules in the formulation is important to see how they are related to the properties and performances of the finished products, such as adhesion to substrate, cohesion strength, improved chemical resistance, etc. This understanding is essential to avoid the unnecessary waste of manpower, energy and expenses involved in experimenting with thousands of formulas simply to find one that works without knowing why it worked.
By the same token, this knowledge is essential to identify and solve any technical problems, which may appear in the course of application. No problem can be solved unless it is correctly defined.
In waterborne adhesive formulations, a blend of polymers is used for economic reasons or to attain a better balance of properties for a specific end use. Each polymer in the blend plays a specific role in determining the end properties.
It is the intention of this article to study the dispersions of pigments and fillers containing different ions in waterborne latices in which the polymer contains ionizable functional groups. Here we report the effect of the metal ion-containing pigments and fillers on the charge density of the waterborne adhesive and coating due to the ion-polymer interactions. Also, the practical impact of these changes on the adhesive properties of the system is discussed.
Experimental
An acrylic-based adhesive formulation developed at Clifton Adhesive was selected, and from this acrylic system, samples of different pH were made by adding different amounts of ammonia. Also, from the same system, individual samples were made by dispersions of fillers containing calcium, magnesium, barium and aluminum ions. The amount of the filler was kept constant for all individual samples at the 10% level.We prepared 1-inch-wide steel substrates by rinsing them in toluene and drying them in an air-circulating oven. To measure adhesion bond strengths to the steel substrate, each solution was then applied to the substrate at a 1-mil thickness. The wet lamination between top-coated steel substrates and cotton cloth was prepared by pressing the coated-steel substrates against the 1-inch-wide cotton substrate and allowed to dry. The peel strength was measured for each prepared laminated sample using Scott Tester Model 3X after allowing the sample to age for 48 hours.
In order to obtain information about the molecular orientation and conformational changes of the polymer on the substrate, we have modified the Buck Scientific PLC-11M single-reflection prism-cell accessory by replacing the reflecting prism cell with a steel substrate. The steel substrate was cut into sizes to fit the reflection accessory. A Buck Scientific Model 500 spectrometer was used, and background scans taken with the accessory were stored in computer memory for further use.
Knowing the solution concentration and the density of the molecules, thin sample films of each solution were cast on the steel substrate calculated to be 100 Adeg. The cast samples were dried in an air-circulating oven at 120degF, and spectra (400-4,000 cm-1 region) were obtained by reflection technique. The reflection technique has been described elsewhere1,2,3. The data were stored in the computer for further analysis.
Results and Discussion
Polymers with ionizable groups along the chain exhibit properties in aqueous solution, which are quite different from those with non-ionizable structures. When a coiled polymeric molecule is ionized, repulsive forces between the ionized groups become operative and the polymer becomes a polyelectrolyte. Thus, for instance, if one adds alkali such as ammonia to a solution of polyacrylic acid used in this study, part of the carboxyl groups are ionized and the carboxylate groups repel each other electrostatically. With increases in the degree of ionization, the repulsive forces overcome the coiling tendency of the Brownian movement and the polymer opens up and extends.Counter ions, such as metal ions, which exist in pigments and fillers, are believed to interact with polyelectrolytes in solution by two principal modes -- the short-range interaction of the counter-ion condensation onto the polyion and the long-range ion-atmosphere interaction with the polyion4-9. The simple concept of "two-phase" condensation model gives results that help approximate important properties of actual polyelectrolyte solutions, such as in waterborne coating and adhesive systems. Basically, the polyelectrolyte charge-density parameter, j, is
- j = e2 / ekTb
where b is the average axial distance between stoichiometric charge groups on the chain, e is the dielectric constant of the bulk solvent, e is electron charge, k is Boltzman constant, and T is Kelvin temperature.
The value of jC is [ZC]-1, where ZC is the counter-ion charge. Ikagami's10 refractometric, Anderson's11 NMR spectroscopy and Kardan's7,8,9 tracer-ion diffusion and fluorescence spectroscopy experiments support the counter-ion condensation to polyions.
Table I summarizes the results of the peel-strength measurements for the acrylic-based adhesive of different pH used in this study when laminated between steel substrates and cotton cloth. The adhesion bond between the sample and cotton cloth did not fail during the test due to the interlock forces with the cloth. Nor did the cohesion bond in the system fail. Therefore, we would interpret that the measured peel-strength values in Table I are directly related to the adhesion of the system to the metal.
- Table I.
Effect of pH on Adhesion of Acrylic-Based Waterborne to Steel Substrate
pH -- Peel Strength (lb/linear inch)
4.20 -- 4.80
4.50 -- 5.30
5.00 -- 5.20
5.50 -- 5.20
6.00 -- 5.50
6.50 -- 6.00
7.00 -- 6.50
7.50 -- 8.00
8.00 -- 8.50
8.50 -- 8.40
9.00 -- 8.00
9.50 -- 7.00
10.0 -- 7.30

These observations suggest that with counter-ion condensation on polymer occurring at jC, the reduction of the charge along the chain facilitates intra-molecular hydrogen-bond formation at lower j values for polyacrylate copolymers and causes recoiling of the polymer.
Analyzing IR Results
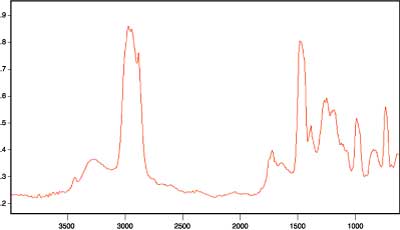
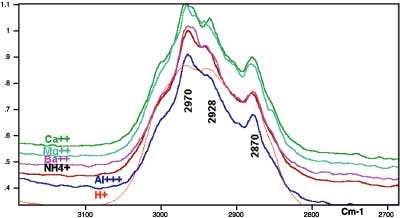
In analyzing the infrared results, our discussions will first center on the structure of acrylic-based adhesive when coated on the metallic surface. Then we will discuss the structural changes as different amounts of ammonia are added to the system to increase the pH.The spectra obtained by reflection, where the polymer is coated on substrate, are useful for structural characterization1,2,3. Figure 2 (page 37) shows the reflection spectra for the acrylic-based adhesive when coated on steel substrates. Although they are not as well understood, the infrared active bands in the high-frequency CH-stretching region can be extremely rich in structural information. Two bands near the 2,970 cm-1 and 2,870 cm-1 region can be assigned to the CH3 asymmetric and symmetric stretching vibrations, respectively, and the bands at 2,928 cm-1 and 2,858 cm-1 are assigned to methylene (CH2) asymmetric and symmetric stretching vibrations, respectively1,12.
The boundary conditions in the reflection technique employed here are such that only incident electromagnetic radiation with polarization in the plane of scattering has significant energy at the immediate surface1,2,3. Therefore, vibrations with components of the transition moments perpendicular to the metallic surface are expected to appear with enhanced intensity while those having transition moments nearly parallel to the surface are expected to appear weakly or not at all. Having such a unique macroscopic frame of reference, one expects these reflection experiments to yield information on structural orientation and their changes, which would otherwise be impossible to obtain.
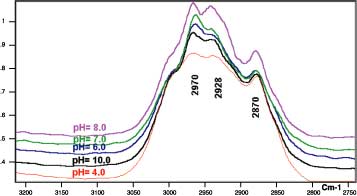
The average of the relative intensity of the symmetric and asymmetric methylene stretching to the relative intensity of methyl-symmetric-stretching vibration for the coated polymer was calculated to increase with increasing pH or decreasing the charge density of the solution. The intensity ratios were found to decrease again upon the addition of the excess amount of amine to the solution.
These results indicate the tendency of the methylene chain to favor the orientation parallel to the metal surface as the polymer expands upon the addition of ammonia up to jC. The addition of excess ammonia (j < jC) will cause the recoiling of the polymer and loss of preferred orientation again. From the infrared data in Figure 3 and adhesion peel results, which are plotted in Figure 1, it appears that the parallel orientation of the methylene chain to the surface will enhance the adhesion of the polymer to the metallic surfaces.
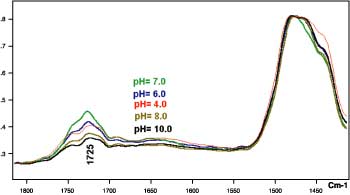
The -CH2 bending vibration absorption band in the 1,400 cm-1 region was used as a reference peak to compare the 1,700 cm-1 intensities for samples of the same system having different solution pH or charge-density values. Several effects can be attributed to this observation. As the carboxyl groups are ionized and the carboxylate groups repel each other electrostatically, the polymer opens up and the C=O group directs itself toward the metal surface and becomes almost perpendicular to the surface. These orientations could be the result of the interaction between the C=O groups with the substrate surfaces, which in turn promotes the adhesion to the substrate. For charge densities lower than jC (pH=10, for instance), the recoiling of the polymer due to the hydrogen results in a loss of preferred orientation for the C=O group and reduction in adhesion to the substrate.
Effect of Fillers and Pigments
In coatings and adhesives, fillers and pigments are used to modify viscosity, end properties and cost of the final film. The defined characteristic of a commercial pigment in a system is its solubility, particle-size distribution, crystal morphology, refractive index and electronic spectra in the visible range, and surface area. Since in waterborne formulations the ion-polymer interactions affect the end properties, the ionic characteristic of the pigment or the filler used in the system becomes extremely important and deserves more exploration.- Table II.
Effect of Different Ions on Adhesion of Acrylic-Based Waterborne System to Steel Substrate
Ion Type -- Peel Strength (lb/linear inch)
H+ -- 5.00
NH4+ -- 7.50
Ba++ -- 8.00
Mg++ -- 12.00
Ca++ -- 11.50
Al+++ -- 9.00
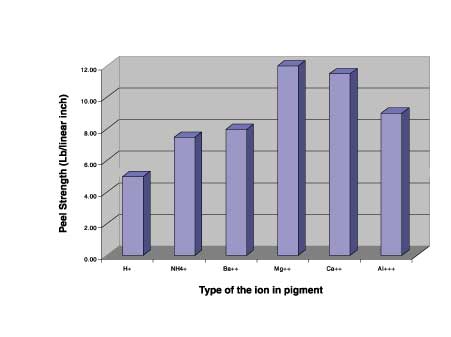
It was at first surprising to observe that metal ions, used here, do not interact with the polymer in the order of trivalent > divalent > monovalent. This finding led us to conclude that in addition to the charge on the ion, the hydrated radii of the ion also affects the adhesion forces.
Conclusions
In waterborne coating and adhesives, the charge density j of the ion-containing polymers, which depends on the pH of the system, is paramount in determining its solution properties. The charge density affects the orientation of the polymer and its adhesion to the surface when coated on the substrate. We have provided some evidences that the adhesion of the waterborne system will reach its maximum values when the charge density of the system is close to its critical value, jC.
The decision of pigment or filler selection for a waterborne coating and adhesive is primarily tied to the type of the interactions, which could exist between the pigment and polymer vehicle. We have also shown that in addition to the charges on the ion, the size of the ion also plays an important role in determining the end properties of coatings and adhesives.
References
1. Kardan, M, Accepted to be published, Rubber Chem. & Tech. (2001).2. Kardan, M. Presented at Adhesive and Sealant Council, 2000 Spring Convention, Las Vegas (April 2000).
3. Kardan, M.; Kaito, A.; Hsu, S.L.; Thakur, R.; and Lillya, C.P. J.Phys.Chem., 19, 1809 (1987).
4. Manning, G.S. Annu. Rev. Phys. Chem., 23, 117 (1972).
5. Zimm, B.H. and LeBert, M. J. Biomol. Struct. Dyn., 1, 461 (1983).
6. Yoshida, N. Chem. Phys. Lett., 90, 207 (1982).
7. Kardan, M. "Interaction of Counter Ions With Polyelectrolytes of Varying Charge Densities", Ph.D. Thesis, Seton Hall University (1984).
8. Ander, P. and Kardan, M. Macromolecules, 17, 2431 (1984).
9. Ander, P. and Kardan, M. Macromolecules, 17, 2436 (1984).
10. Ikagami, A. J. Polymer Sci., A2, 907 (1964).
11. Anderson, G.; Record, M.; and Hart, P. Biophys. Chem.,7, 301 (1978).
Kardan, M.; Reinhold, B.B.; Hsu, S.L.; Thakur, R.; and Lillya, C.P. Macromolecules, 19, 616 (1986).
