Polyurethanes have been often described in literature as one of the most versatile polymers known2,3,4. The reason for such success can generally be explained as the product of two windfalls. First, polyurethanes may be prepared as either cellular or non-cellular products, which allow polyurethanes to find applications in numerous end uses. The second reason for the versatility of polyurethane polymers comes from the abundance of raw materials available to prepare polyurethanes. Strictly speaking, general polymer nomenclature regards a polymer as a polyurethane even if other functional groups are also present. Hence, polyurethanes may be built from starting materials containing many polyester and/or polyether polyols, by reacting them with aliphatic or aromatic isocyanates.
Typically, ester-containing polyols offer abrasion resistance and adhesion promotion while polyether polyols provide low-temperature flexibility and low viscosity. Industrial polyether polyols are generally limited in monomer composition to propylene oxide, ethylene oxide, butylene oxide and tetrahydrofuran. Industrial polyester polyols, many of which are the condensation products of organic acids and alcohols, may be prepared by a great many more combinations of monomers and thus add to the potential versatility of polyurethane products.
Stepan Co. has recently developed several new phthalic anhydride-based polyester polyols suitable for use in the C.A.S.E. industry. In this article, we present the utility of the new polyols designated STEPANOLR PN-110, STEPANOL PD-56, STEPANOL PH-56 and PD-56LV by examining their effects in several C.A.S.E. applications.
Experimental
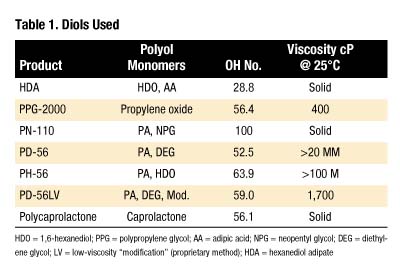
Materials were used as received from the manufacturer. Table 1 lists characteristics of diols used (hydroxyl or "OH" number is expressed in mg KOH/g).All products in Table 1 are polyesters except for PPG-2000, which is a polyether. The following abbreviations are used:
- HDO = 1,6-hexanediol
- PPG = polypropylene glycol
- AA = adipic acid
- NPG = neopentyl glycol
- DEG = diethylene glycol
- LV = low-viscosity "modification" (proprietary method)
- HDA = hexanediol adipate
Isocyanates included in this study were Mondur M (pure MDI, or 4,4'-diphenylmethane diisocyanate, equivalent weight = 125 g/eq), Mondur MR (polymeric MDI, equivalent weight = 131 g/eq), Mondur CD ("liquid pure" or carbodiimide-modified MDI; equivalent weight 143 g/eq) and Desmodur W (131 g/eq). Catalysts used in formulation work were DMDEE (dimorpholodiethyl ether) and DBTDL (dibutyl tin dilaurate).
Formulations for isocyanate-rich prepolymers were calculated to give a specified weight percent of isocyanate ("%NCO") assuming the hydroxyl equivalent weight of each glycol used was the hydroxyl number for a glycol divided into 56,100.
Prepolymers to be used as liquid adhesives and in elastomers were synthesized at 5, 10 and 15% target %NCOs by combining the required amounts of desired polyols and Mondur M in a clean can and heating the contents to approximately 70 deg.C under agitation and a nitrogen pad for approximately two hours. Products were finally sealed in the can under a nitrogen blanket overnight before determining the product %NCO (ASTM D 2572) and viscosity.
Selected prepolymers were evaluated as one-part moisture-cure adhesives by first blending in 0.25% by weight of DMDEE catalyst into prepolymers studied. Laminate assemblies were then made by spreading approximately 14 grams of catalyzed prepolymer to Lauan wood measuring 1-ft x 1-ft x 3?-in thick. Fiberglass-reinforced plastic (FRP) was next placed on top of the treated Lauan surface, and the assembly was clamped together at 40 psi of pressure to cure overnight under ambient conditions.
Test specimens were prepared by trimming the bonded Lauan-FRP assemblies to 1-in x 2-in rectangles and bonding the opposite untreated surfaces to matching aluminum T-bars with epoxy adhesive. The final assemblies were tested with an Instron Model 1123 test machine with a separation of 0.5 in/min following at least one-hour storage at a test temperature.
Elastomers were made by hand-mixing selected prepolymers with sufficient 1,4-butanediol for a "103 Index" formulation (i.e., 1.03 times the isocyanate weight required to affect a stoichiometric balance between a mixture of polyols and an isocyanate). To facilitate cure, DBTDL at 0.1% of the total polyol and isocyanate blended weight was quickly mixed in by hand and the resulting liquid was immediately cast into molds preheated at 120oC. Molds were maintained at 120 deg. C for an additional hour and then placed in ambient conditions for at least four hours before parts were de-molded. Parts were allowed to stand under ambient conditions at least one week before evaluating for tensile strength and elongation (ASTM D 638, Type I) and Shore A hardness (ASTM D 2240).
Prepolymers for polyurethane-reactive hot melt (PURHM) adhesives were prepared in a similar manner, except that the target %NCO was 2% and reaction times were about one hour at 120 deg. C. The procedure for determining %NCO of these products was modified by first dissolving a weighed amount of a PURHM in 25 - 100 ml of dry, warm ethyl acetate and then proceeding with the ASTM D 2572 technique.
Hot melt adhesives were evaluated for green strength by recording the Instron force required to separate cubic-inch pine blocks bonded for 30 seconds with a 295 gram weight serving as compression. The actual bond line was prepared by first applying a drop of molten PURHM at 120 deg. C onto the room-temperature surface of one pine cube and quickly drawing the drop into a thin film to cover the entire cube face with a wooden tongue depressor. The face of another untreated pine cube was aligned with the treated face to effect the bond line and compressed as above. Green-strength times were measured as the delay between contacting the pine blocks and the start of the actual separation of a bonded pair of blocks. Blocks had pre-drilled holes to accommodate eyehooks, which allowed mounting on Instron clamps.
"Quick Stick" testing was done by applying a PURHM (at 120 deg. C) to a tongue depressor and bonding the depressor to the corresponding substrate. Following three days at ambient conditions, these assemblies were separated by hand and examined for the mode of bond failure.
Polyurethane dispersions (PUDs) were prepared by a typical multi-step process. First, a mixture of a selected polyol and dimethylolpropionic acid (DMPA) was reacted with an excess of Desmodur W at 80 deg. C - 95 deg. C in the presence of N-methyl-2-pyrrolidone (NMP) until a target %NCO of approximately 4% was reached (2 - 4 hours). DBTDL (100 ppm) was used as the catalyst in the prepolymer synthesis. The resulting prepolymer was cooled to 60 deg. C and then neutralized with a stoichiometric amount of triethyl amine (TEA) based on the amount of DMPA used. The neutralized prepolymer was cooled to room temperature and dispersed in deionized water under high shear of 3,000 - 5,000 rpm. Hydrazine hydrate was added to the mixture, and the dispersion was stirred for 30 minutes under high-shear conditions. The NCO/NH2 equivalent ratio was 1.05.
PUD dispersions were characterized using a NICOMP 370/Autodilute Submicron Particle Sizer and the pH Metrohm Potentograph E 536. Films were cast using a 10-mil draw-down bar and allowed to cure for seven days at ambient conditions. The pencil hardness was measured using ASTM D 3363-92A. Quick-stick testing was done in a way similar to that described above except that the adhesive was allowed to cure for four days at ambient conditions.
Results and Discussion
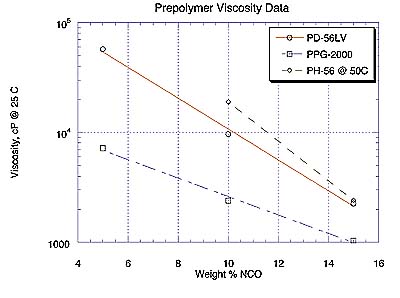
Prepolymer ViscosityPrepolymer viscosity data are most conveniently represented in Figure 1 as %NCO plotted against the logarithmic ordinate of viscosity (in cP at 25 deg.C) since these transformed relationships tend to be linear. Prepolymers of PD-56 and PN-110 were in excess of 100,000 cP due to the higher viscosity of the starting materials and were therefore not measured. Note that PH-56 data were recorded at 50oC for 10% and 15% NCO products. The viscosity of the 5% NCO prepolymer of PH-56 was quite high and is not displayed here.
As expected, prepolymer viscosities in Figure 1 are negatively correlated with %NCO. Note prepolymer viscosities of PD-56LV, a new 'low-viscosity" polyester diol, and PPG-2000 approach one another as %NCO in their respective prepolymers increases.
Adhesives and Elastomers
Prepolymers of pure MDI were selected for this study for their utility in both adhesives and elastomers. When catalyzed with 0.25% DMDEE by weight to assist moisture curing, the ability of these prepolymers to act as liquid PUR adhesives was studied. Alternatively, the same prepolymers were extended with 1,4-butanediol and catalyzed with DBTDL to prepare elastomers.
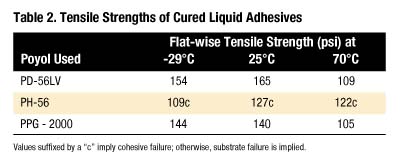
For the liquid adhesive study, due to the higher viscosity observed at the lower %NCOs, only 15% NCO prepolymers were used. Lower viscosity products are usually preferred to improve substrate wet-out and final bonding.
Information from this study suggests liquid MDI prepolymers of PH-56 are not candidates of choice for Lauan to FRP bonding. Possible explanations include the high viscosity of these prepolymers or the chemical compositions of this diol are not optimum for bonding these materials.
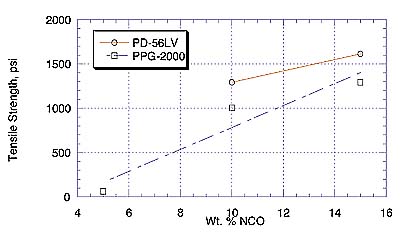
Comparison of PPG-2000- and PD-56LV-MDI adhesives shows substrate failure (Lauan) at all temperatures studied. Here, the flat-wise tensile strength values are higher for the PD-56LV than the PPG analogues, in spite of the fact that the PD-56LV prepolymer is more viscous. It is quite possible that less viscosity is useful to a point, below which adhesion quality depends more on the quality of the polymer backbone. The reason for a relatively high tensile strength without substrate failure with the PH-56 prepolymer remains unclear.
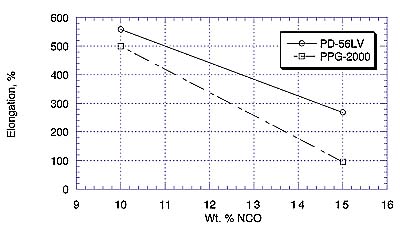
Elastomers based on PD-56LV again outperformed analogues made from PPG at the corresponding %NCO in terms of exhibiting higher tensile strength and elongation. This may be due to a chemical difference with polyester vs. polyether, or to the fact that PD-56LV does not contain the same type of unsaturation (as allyl alcohol) as is generally found in higher-molecular-weight polypropylene glycols5.
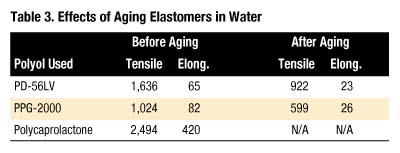
The extension of this work was to compare the hydrolysis resistance of the elastomers made with PD-56LV, PPG-2000 and a polyester polyol generally considered one of the more resistant to hydrolysis, polycaprolactone6,7. A series of "one-shot" elastomers was made by blending polyol, 1,4-butanediol (30 parts per hundred parts polyol), Mondur CD (103 index) and DBTDL catalyst by hand, casting into preheated molds and curing as with the other elastomers.
Water aging of these one-shot elastomers was accomplished by soaking cured dog-bone specimens in separate cans at 88 deg. C. After 29 days, parts were removed from the water, blotted dry and dried in a convection oven at 70 deg. C overnight. Samples were allowed to equilibrate under ambient conditions at least three hours prior to testing. Table 3 shows the results.
Percentage retention of both tensile strength and elongation are nearly identical for the PD-56LV and PPG-2000 elastomers (tensile retained = 56% and 58% and elongation retained = 35% and 32%, respectively), whereas the polycaprolactone-based elastomer was too brittle to evaluate for these properties. All three elastomers exhibited 95 - 96 Shore A hardness before aging and 94 - 97 Shore A hardness after aging. Thus, elastomers prepared with the polyester PD-56LV exhibit the same magnitude of retention of tensile and elongation as a PPG analogue.
Bonding Properties of PUR Hot Melt Adhesives
A series of PUR hot melt adhesives (or PURHMs) were prepared by blending polyols at 120 deg. C and reacting with pure MDI to approximately 2% NCO. Characterization of the products was made in terms of 120 deg. C viscosity, physical properties of open time and initial or green strength, and the ability to bond pinewood to various surfaces.
There is no standard test for green strength. This is unfortunate, as green strength is a common term in the adhesives industry as an attempt to describe the amount of "strength" an adhesive first exhibits, especially in resisting memory of bonded substrates. In this work, we adopted a technique of applying a film of molten hot melt adhesive to wooden blocks, pressing them together under specified conditions and then recording the amount of force required to separate them with a strain rate of 10 inches (25.4 cm) per minute.
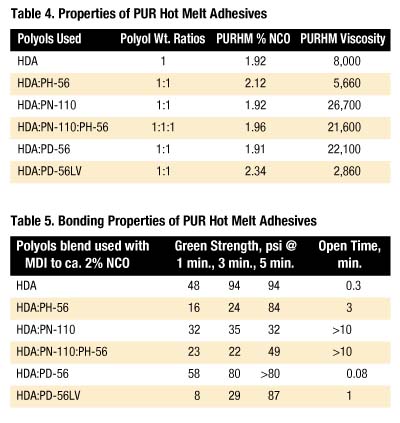
Table 4 lists the polyol combinations used to prepare PURHMs along with %NCO and viscosity (cP @ 120 deg. C).

In general, PUR hot melt adhesives with the shortest open times have the highest green strength. Only the PUR hot melt adhesive containing PD-56 is opaque when molten; all other PUR hot melts appear clear. The shorter open time observed for the PD-56 product may indicate the role of compatibility; an incompatible melt allows the HDA segment to segregate and quickly crystallize when cooled, whereas compatible systems solvate HDA, thereby retarding crystallization. Note that this delay does not necessarily compromise green strength.
Quick-stick testing was done on four different substrates as shown in Table 6. PH-56 and PD-56LV give the best bonding characteristics with the substrates evaluated. It is interesting to note that even though PN-110 was added to the PH-56 formulation, it did not affect the quick-stick bonding characteristics.
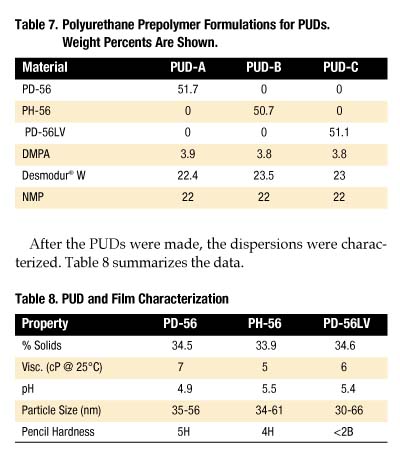
PUD and Film Characterization
To gain an understanding of how these polyols perform in polyurethane dispersions (PUDs), formulations were prepared from PD-56, PH-56 and PD-56LV. Since many factors influence PUD properties, simple, non-optimized formulations were examined (Table 7).
After the PUDs were made, the dispersions were characterized. Table 8 summarizes the data.
All three PUDs have a white opaque/blue hue appearance, did not precipitate after seven days in a 50 deg. C oven and have very low viscosities at ambient temperatures. As shown in Table 8, the particle size for each PUD has the same range. However, PD-56 and PH-56 provide a much harder film than PD-56LV. One unexplained result was the low pH associated with each sample.
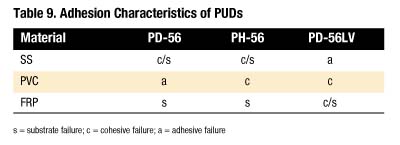
A quick-stick test was used to evaluate the adhesive characteristics of each PUD. Table 9 summarizes the results. As shown, PH-56 displays the best bonding profile of the three.
Conclusions
Several new polyester polyols have been developed and explored for utility in polyurethane C.A.S.E. applications. Adhesives based on PD-56LV provide flat-wise tensile strengths exceeding results for the analogous PPG-based system.Elastomers using PD-56LV as the major (soft segment) polyol and extended with 1,4-butane diol generally demonstrated higher tensile strength and elongation values as compared with elastomers using conventional polypropylene glycol as a soft segment. Not only has this been demonstrated for both one- and two-shot methods, but also the retention of these properties is similar after a one-month soaking in hot water. In addition, property retention of the PD-56LV elastomer after water soaking is superior to that of a similar polycaprolactone-based elastomer. As previously reported, the ortho positioning of ester groups in PD-56LV is likely responsible for this superior resistance to degradation by water1.
Also, PD-56LV demonstrates utility in both PUR hot melts and PUDs. In a hot melt adhesive formulation with hexanediol adipate, PD-56LV at the level used provides an excellent bonding profile, high green strength and a relatively short open time. It also has been successfully used in a PUD formulation, generating a stable dispersion and a soft film.
The other three polyols, PH-56, PD-56 and PN-110, have been shown to provide versatility in PUR hot melt applications. When combined with hexanediol adipate, PH-56 generates a hot melt with excellent bonding characteristics and a short open time; PD-56 provides similar results. PN-110 can be used as an agent to extend open time in a PUR hot melt adhesive.
Both PH-56 and PD-56 made stable polyurethane dispersions. The PUDs made with these polyols give hard films and small particle sizes. Of the three PUDs made, PH-56 exhibited the best bonding profile.
In summary, several ortho-phthalate polyols have been successfully used in multiple C.A.S.E. applications. The inherent hydrolytic stability of these ortho-phthalate-based polyols coupled with the economical performance benefits provide performance advantages for many C.A.S.E. applications.
This article is based on a presentation made at the Polyurethanes Conference 2000 sponsored by the Alliance for the Poyurethanes Industry in Boston last October. Additional information on STEPANOL polyester polyols is available from Stepan Co., 22 West Frontage Road, Northfield, IL 60093; call 800-745-STEP (7837) (Technical Service), fax 847-441-4166, e-mail hhalpert@stepan.com or visit the Web site www.stepan.com. Or Circle No. 75.
References
Hillshafer, D. K.; O'Brien, M. E.; Knutsen, K. J.; Proceedings of Polyurethane Expo, 1996, pp. 530-537.Skeist Inc., Polyurethanes IV, Skeist Inc., Whipppany, N.J., 1994, p. 1.
Buist, J. M. and H. Gudgeon, Advances in Polyurethane Technology, John Wiley and Sons, Inc., 1964, p. 1
Oertel, Gunter, Polyurethane Handbook, Hanser Publishers, 1994, pp. 1 - 3.
Herrington, Ron and K. Hock, Flexible Polyurethane Foams, The Dow Chemical Co., Midland, Mich., 1997, p. 2, 8.
Union Carbide Corp., "Tone Polyols for High Performance Urethane Elastomers and Adhesives" (Technical Brochure), UCC, 1993, pp. 4, 18
Union Carbide Corp., "Tone Monomer EC" (Technical Publication), UCC, 1987, pp. 1, 11.