Although the phenomenon of carbon-black reinforcement has been known for almost a century, the nature of the mechanism is not yet fully understood and is a subject to debate. Therefore, a considerable amount of interest exists in characterizing the nature of carbon-black binding to the polymer and the associated properties.
Even though the existence of different degrees of reinforcement with different types of carbon black has been previously established for natural rubber, experimental evidence suitable for microstructural analysis is rare. Most of the studies, including modulus or tensile-strength measurements, thermal measurements, or optical microscopy, are not actually directed at obtaining the details of the microstructures.
The Fourier Transform Infrared (FT-IR) external-reflection experiment by Kardan and coworkers1,2,3 has revealed that, when a polymer is coated on a substrate, each structural sub-unit of the polymer has a defined orientation on the external surface. The orientation of the polymer on the surface and its adhesion to the substrate depend on its conformational changes, which are influenced greatly by the interaction of the polymer with the media 4,5.
In this study, we have used infrared-reflection spectroscopy as a primary technique to analyze the polymer structural changes in the carbon black/rubber composites when coated on metallic substrates. The correlation of the infrared results with the measured lap shears for the composites, when laminated between two metallic substrates, can be useful in the elucidation of the mechanism for carbon-black reinforcement. Because of the similarity in structure between natural rubber and many other elastomers, the structural information we deduce from this analysis can be transferred to other elastomer/carbon-black adhesive and sealant systems.
Composites of synthetic natural rubber (SNR) 99% cis-1,4 polyisoprene (Natsyn 2200) with carbon black and composites of natural rubber (NR) (White Crepe) with carbon black were prepared by mill-compounding the carbon into the rubber by a specific technique developed at Clifton Adhesive, under the exact same conditions. Eight different grades of carbon blacks with particle sizes ranging from 18 to 90 nm were used in this study. For all prepared composites, the carbon-black level was kept at 5 phr (5 parts carbon per hundred parts of rubber).
Solutions of each composite in toluene were prepared separately. In order to obtain information about the molecular orientation and conformational changes of the polymer on the substrate, each solution was coated on a steel substrate (all coatings having an equal thickness). Infrared-reflection spectra of each coated sample were taken. The external reflection technique has been discussed elsewhere1. The data were stored in a computer for further analysis.
A topcoat of a primer PC4426, developed at Clifton Adhesive to maximize the anchorage of subsequently applied adhesive to metal, was applied to 1-inch-wide steel substrates at 0.025-mm (1-mil) thickness and allowed to dry at room temperature overnight. Each solution was then applied to two top-coated substrates at 0.1-mm (4-mil) thickness and air dried. The laminations were prepared by pressing two coated substrates against each other. The lap shear was measured for each lamination, after allowing it to age for 48 hours at room temperature, using Scott Tester Model 3X.
Results and Discussion
One of the main goals of our analysis is the characterization of the structural aspects of natural rubber in the composites with different grades of carbon black. It is generally accepted that the particle size, aggregate structure and surface area of the carbon black are important factors in reinforcing the rubber. However, it is not clear what structural differences exist between the rubber before and after the reinforcement. Nor is it clear how structural disorder is introduced when different types of carbon black are compounded into the rubber. These structural changes are associated with the interaction of the carbon black and the rubber.Although the loading level for different grades of carbon black is a critical factor in achieving the degree of rubber modification for desired end-use application, this level was deliberately kept constant at 5 phr for all carbon-black grades in our studies. This gave us the assurance that we had a reasonable understanding of the interactions between the polymer and the carbon black.
In the lap-shear experiment, the adhesive bond between the composite and the primer, PC 4426, did not fail during the test, nor did it fail between the primer and the steel substrate. Therefore, we would interpret that the measured lap-shear values are directly related to the cohesion forces in the composites.
Nitrogen surface area (specific surface, determined by nitrogen-absorption capacity) and DBP (dibutyl phthalate absorption, which is a measure of carbon-black aggregate structure) are both measures of the carbon black’s surface area. Carbon black is composed of clusters of fused, prime particles called primary aggregates. If the primary aggregate is composed of many prime particles, with considerable branching and chaining, it is referred to as a high-structure black.
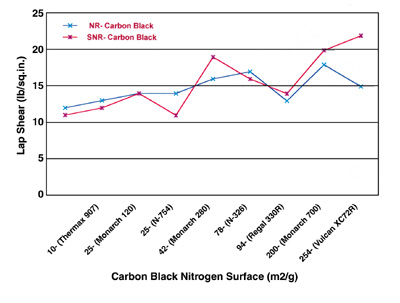
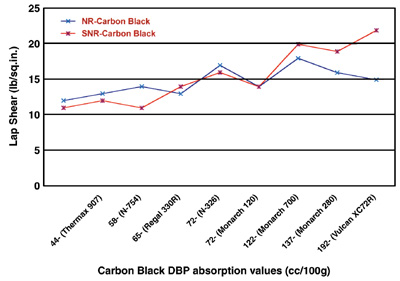
From Figures 1 and 2, it can be seen that the lap shear increases with increasing both carbon-black nitrogen surface area and DBP absorption values. It appears that the increases are more pronounced and correlated with increasing DBP absorption values than they are with increasing nitrogen surface areas.
The plots in Figures 1 and 2 indicate that the properties of both primary aggregates and spherical particles comprising them are important controlling factors in carbon-black performance. Higher surface areas, as imparted by finer prime-particle blacks, require more energy for wetting and dispersion. They also tend to “tie up” more rubber per unit weight of carbon, resulting in stiffer compounds than with coarser blacks.
Based on our analysis of the infrared results, our discussions will first center on the structure of the natural rubber when coated on the metallic surface. Then we will discuss the structural changes as different grades of carbon black are milled into the rubber. The task is made easier by analyzing well-assigned infrared vibrations, which are localized in nature with well-defined transition moments.
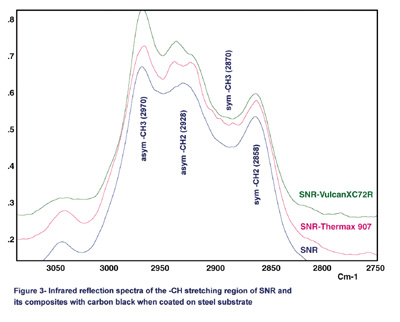
Figure 3 shows the comparison of the reflection spectra for SNR and its composites with carbon black coated on steel substrates. In this figure, Vulcan XC72R (XC72R) and Thermax 907 are shown as examples because of the large differences that exist between their particle sizes and surface areas. Also, the lap-shear results indicate that the XC72R is a strongly reinforcing carbon black and Thermax 907 is a weakly reinforcing carbon black.
Although they are not as well understood, the infrared active bands in the high-frequency C-H stretching region can be extremely rich in structural information. Two bands near the 2,970 cm-1 and 2,870 cm-1 region can be assigned to the CH3 asymmetric and symmetric stretching vibrations, respectively. The bands at 2,928 cm-1 and 2,858 cm-1 are assigned to methylene (CH2) asymmetric and symmetric stretching vibrations, respectively.
We have noted that the calculated ratio of 1,450 cm-1 band to 1,433 cm-1 in the spectra will slightly increase with increasing carbon black DBP absorption values. The interaction between carbon black and the polymer, therefore, results in a loss of preferred conformation, conceivably because the polymer forms a strongly absorbed surface layer.
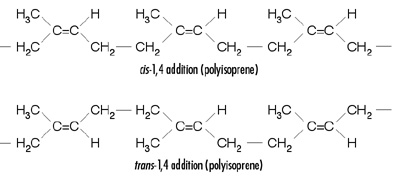
From the infrared data, it appears that a high-structure carbon black such as XC72R can be absorbed on the elastomer surface and promote mostly trans conformation for the rubber structure. It is not unlikely that carbon-black primary aggregates or high-structure aggregates reinforce the rubber structure by entanglement and mechanical interlocking forces. Also, both infrared and lap-shear results indicate that the carbon-black reinforcement is more effective for SNR, which is 99% cis conformation, than with NR, which is not fully cis conformation.
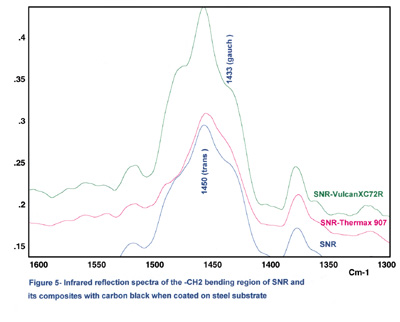
Several reasons can be attributed to this observation. Carbon blacks contain chemical complexes on their surfaces, either resulting from oxidation during manufacture or from deliberate after-treatment of the surface. These complexes are chemisorbed oxygen groups in the form of carboxy, quinone, lactone and phenolic groups.
It is unlikely that the surface of inactive carbon black Thermax 907 shows a certain chemical reactivity during aging while active carbon XC72R does not show this reactivity. Therefore, we would interpret that the inactive carbon black, Thermax 907, leads to an increased amount of chemical crosslinks within the elastomeric polymer network that will cause a significant decrease in the CH2 bending intensity. On the other hand, the reinforcing carbon black will not participate in any crosslinks within the network.
This conclusion is especially consistent with the recently reported 1H Nuclear Magnetic Resonance (NMR) relaxation results for natural rubber filled with carbon black. The relaxation results in the study indicated that in the process of curing natural rubber, active carbon black led to a higher reduction of crosslink densities than inactive carbon black10.

Conclusions
The strength-related properties, such as cohesion forces in adhesives and sealants, are enhanced by higher carbon-black surface area. The degree to which carbon particles bind together in aggregates (primary structure) and the agglomeration of aggregates due to Van der Waals forces (secondary structure) play an important role in reinforcing the rubber and enhancing the cohesion forces in natural rubber-based adhesives and sealants.We have provided some evidence that reinforcing carbon black will not participate in any crosslinks within the elastomer network. Most likely, the carbon black primary or high-structure aggregates reinforce the rubber structure by formation of entanglement network or mechanical interlocking forces. The infrared and lap-shear results indicate that the reinforcement mechanism promotes the trans- conformation in the rubber structure. We have also concluded that the active carbon black is more effective when the rubber already exists in cis- conformation as opposed to trans- conformation.
This article is based in part on a presentation made at the Adhesive and Sealant Council 2000 Spring Convention, Las Vegas, April 2000. Additional information on industrial adhesives and coatings is available from Clifton Adhesive, Inc., Burgess Place, Wayne, NJ 07470; phone: 973-694-0845; fax: 973-694-5678.
