
Hard-to-bond plastics such as acetals, polyethylenes, fluoropolymers, polypropylene and TPVs are used in virtually all industries due to their low cost, flexible design capabilities, and superior performance properties. Many assembly methods exist for joining similar and/or dissimilar plastics — from mechanical to chemical methods. Adhesive assembly provides unique benefits such as the ability to bond and seal, even distribution of stress across a joint, rapid fixture and cure times, gap filling abilities, and easy automation.

Defining Hard-to-Bond Substrates
Hard-to-bond substrates feature no functional sites or surface roughness onto which an adhesive can secure itself. They are characterized by either linear or branched carbon chain polymers, low surface energies, low porosity, and non-polar or non-functional surfaces.Thermoset plastics are resins that cannot be melted or reformed once polymerized, and include polyester, phenolic and epoxy resins. Thermoplastics are resins that can be reflowed under heat following final processing, and include acrylonitrile butadiene styrene (ABS), polyamide (nylon), polycarbonate, and polyolefins such as polyethylene and polypropylene.
Low-density polyethylene (LDPE) is a semi-crystalline thermoplastic produced by way of a free-radical-polymerization reaction. Although LDPE typically has lower strength and hardness properties, it offers numerous benefits including flexibility, clarity, and enhanced impact and stress cracking resistance.
High-density polyethylene (HDPE) is similar to LDPE in the polymerization process used to obtain the resin. However, the density of the polyethylene increases, resulting in higher strengths, increased hardness, and enhanced chemical and abrasion resistance. Common uses for polyethylenes include packaging containers for food, chemicals, and automotive fluids; films used for shrink wrapping, packaging and drum lining; and various other medical device, electrical component, and appliance applications.
Polypropylene (PP) is a crystalline thermoplastic known for its excellent thermal- and chemical-resistance properties. In addition to high moisture resistance, PPs are often selected for their good mechanical properties. The primary disadvantage of PP is its low-temperature impact resistance, a property directly related to the polymer’s degree of crystallinity. Like polyethylenes, PP is commonly used in the packaging, appliance, medical, electrical and fiber industries.
Fluoropolymers such as Teflon are highly crystalline thermoplastics produced using a free radical polymerization reaction. Fluoropolymers are characterized by superior thermal and chemical resistance, low coefficients of friction, excellent weatherability, and low flammability. With typical service temperatures of approximately 500 degrees F, fluoropolymers work well in high-temperature applications in the electrical, mechanical, chemical, home appliance and packaging industries. The high per-pound cost of some grades of fluoropolymers is a potential drawback of these substrates over other thermoplastics.

Classified as thermoplastic elastomers, thermoplastic vulcanizates (TPVs) are sometimes considered hard-to-bond plastics. Formed by combining polypropylene with a vulcanized rubber, TPVs offer excellent thermal, weathering and chemical resistance. Because of their inherent flexibility, TPVs exhibit excellent flexural resistance, abrasion resistance and tensile strength properties. These materials are used in the automotive, construction, electrical and medical industries, among others.
Adhesives for Hard-to-Bond Plastics
Hard-to-bond plastics are most often assembled using adhesives.1While adhesives are the most versatile assembly method for plastics, capable of joining 36 types of plastics, only a few industrial adhesives offer consistently high bond strengths on hard-to-bond plastics. Cyanoacrylate, light-curing cyanoacrylate, hot-melt and occasionally light-curing acrylic adhesives have exhibited high bond strengths on typical difficult-to-bond substrates including PE, PP, acetal, fluoropolymers, and TPVs. A new breed of acrylic adhesive is also designed for use with hard-to-bond plastics.
Cyanoacrylate adhesives are polar, linear molecules that undergo an anionic polymerization reaction. A weak base, such as moisture, triggers the reaction causing the linear chains to form. The products are maintained in their liquid form through the addition of weak acids that act as stabilizers. Many cyanoacrylate formulations are available with varying viscosities, cure times, strength properties and temperature resistance.
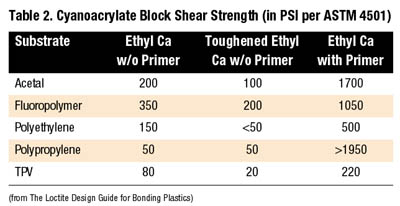
Rubber-modified cyanoacrylate formulations offer greatly improved peel and impact strengths over standard cyanoacrylates. The compounded rubber added to ethyl formulations slightly increases fixture time to 30 seconds to two minutes.
Thermally resistant ethyl cyanoacrylates can withstand continuous exposure to test temperatures up to 250 degrees F. Available in both black and clear versions, these modified toughened cyanoacrylate products also have increased fixture times.
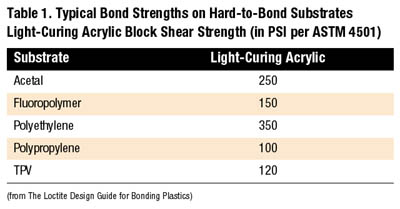
Although light-curing acrylics do not offer the significant performance strength of cyanoacrylates on hard-to-bond substrates, they do offer moderate performance on several of the cited plastics including acetal and polyethylene (see Table 1).
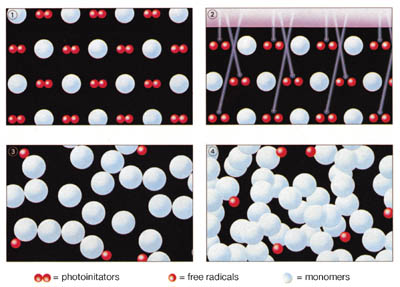
Light-curing acrylics cure by way of a free radical reaction to form thermoset resins when exposed to light of the appropriate wavelength and intensity (Figure 2). Like cyanoacrylates, light-curing acrylic adhesives are available in a range of viscosities from low (~ 50 cP) to thixotropic gels. In addition, light-curing adhesives vary in final cured form from hard and glass-like to soft and flexible resins.
With these adhesives, light must reach the entire bondline to achieve full cure. Any adhesive remaining in shadowed areas will remain liquid. Light-curing acrylics fixture rapidly and cure in as little as five seconds following light exposure. Because the final resins are thermoset plastics, light-curing acrylics offer enhanced thermal, chemical and environmental resistance over cyanoacrylate adhesives. Table 1 shows typical bond strengths on hard-to-bond substrates using a standard ultraviolet and/or visible curing adhesive.
Light-curing cyanoacrylates are ethyl-based products that have photoinitiators added to the formulation, allowing them to fixture rapidly on exposure to low-intensity light, and to cure in shadowed areas. Physical performance characteristics of these adhesives are similar to those of traditional cyanoacrylates. Light-cure cyanoacrylates minimize blooming/frosting since exposed, uncured cyanoacrylate can be immediately cured using ultraviolet and/or visible light. While traditional cyanoacrylates cure to a maximum of 0.010 in., cure depths in excess of 0.5 in. have been achieved with light-curing cyanoacrylates. These adhesives are compatible with primers for hard-to-bond plastics. The bond performance of light-curing cyanoacrylates on difficult-to-bond substrates is similar to that of traditional ethyl cyanoacrylates.
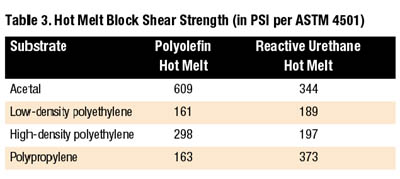
Polyolefin hot melts provide good resistance to moisture as well as excellent resistance to polar solvents, acids, bases and alcohols. The polyolefin family of hot melts offers superior adhesion to polypropylene when compared to other types of hot melts.
Reactive urethane hot melts perform well on hard-to-bond plastics. While most traditional hot melts are thermoplastic resins that can be repeatedly reheated, reactive urethanes form thermoset plastics when fully processed. Although initial strength develops a bit slower than traditional thermoplastic hot melts, fully cured reactive urethanes are superior performers. In addition to offering good bond strengths to difficult-to-bond materials, reactive urethanes process at temperatures of approximately 250 degrees F, as much as 200 degrees F cooler than EVA, polyamide, and polyolefin hot melts.
A new two-part acrylic-based adhesive offers superior performance on materials such as polyethylene and polypropylene. The two-part acrylic offers tenacious bond strength without surface preparation, creating both mechanical and chemical attachment to mated surfaces. In addition to its performance on hard-to-bond plastics, the acrylic adhesive provides in excess of 1,000 PSI on many traditional metal and plastic materials, including polycarbonate, PVC, steel, and aluminum.

Surface Preparation Methods
To enhance adhesion, hard-to-bond materials require surface preparation prior to joining. Surface preparation methods for hard-to-bond plastics include both chemical and physical treatments designed to increase reactivity and roughness on the surface of the substrate. Common methods include plasma or corona treatment, flame treatment, chemical etching, or surface priming.Plasma treatment is used on a variety of substrates, including polyolefins and polyester. A gas such as oxygen, argon, helium or air is excited at low pressure, resulting in the production of free radicals. The ions generated bombard the substrate surface and form reactive groups that increase surface reactivity and wettability.
One major drawback with plasma treatment is its potentially short shelf life. Most substrates treated with plasma must be assembled within a very short time period since the reactive surface is re-exposed to air and rapidly reverts back to its normal state.
Plasma treatment is frequently used with cyanoacrylate, light-curing acrylic and light-curing cyanoacrylate adhesive technologies. Since each plasma gas imparts different characteristics onto a substrate, end users should consult with their adhesive and substrate suppliers to determine the optimum gas for the materials used.
Like plasma exposure, corona treatment is most frequently used on polyolefin substrates bonded with cyanoacrylate or light-curing acrylic adhesives. The treatment generates reactive groups that serve as potential bonding sites for the adhesive, and result in a significant enhancement of bond strength.
Chemical treatment methods such as chromic acid etching are often used on polyolefins and acetals. Like other surface treatment methods, chromic acid etching adds reactive species to a surface. Because it can be hazardous, chemical treatment is used on a limited basis to treat a variety of substrates prior to bonding with cyanoacrylate and light-curing acrylic adhesives.
Handling and storage of hazardous substances are potential disadvantages of chemical treatments. Surface changes are controlled by two variables: the solvent selected and the overall exposure time.
In flame treatment, various reactive groups such as hydroxyls, carbonyls and carboxyls are introduced to bonding surfaces through an oxidation reaction when the substrate is exposed to flame. In addition, flame treatment increases surface energy of the substrate surface, allowing for better wetting. Flame treatment is commonly used on polyolefins and polyacetals, and is most frequently used when bonding with cyanoacrylate adhesives.
Surface roughening results in mechanical interlocking sites and causes bond strength to increase dramatically. A surface roughness of approximately 63-125 micro-inches is often used as a guideline for assemblies that are to be bonded with adhesives. Surface roughening will significantly increase the bond strength of most adhesive technologies, and is highly recommended for both hard-to-bond and traditional substrates.
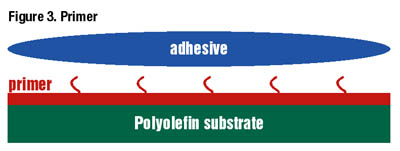
A variety of substrates exhibit enhanced bond strengths following treatment with primers, including polyolefins, acetals, fluoropolymers and TPVs. Although using a surface primer is often viewed as a two-step bonding process, primers can be applied with little to no capital expenditure, and many remain active on substrates for more than eight hours.
Polyolefin primers are frequently used on hard-to-bond substrates joined with traditional and/or light curing cyanoacrylate technologies. Introducing the active species to the substrate surface generally increases bond strength three-fold for traditional ethyl cyanoacrylate adhesives.
References:
1 “Engineer’s Guide to Plastics” published by Materials Engineering.The Loctite Design Guide for Bonding Plastics, V. 2, LT-2197A.
The Loctite Design Guide for Bonding Rubber and Thermoplastic Elastomers, V. 1, LT-2662.
Handbook of Plastics Joining, A Practical Guide, 1997 by Plastics Design Library, published by Plastics Design Library, Norwich, NY.
