
Hydroxyl-terminated polybutadiene diols are now available that can be employed to prepare novel soft TPUs. These hydrophobic elastomers are suitable for adhesive and sealant applications requiring excellent hydrolytic stability, low-temperature flexibility, and outstanding resistance to aqueous acids and bases.
Development of soft grades of TPUs (having hardness ranging from 70-85 Shore A) has been reported among the latest trends in the TPU market. The main interest of researchers is focused on the polyol component[2].
Polybutadiene polyols with a functionality of 2.0, designated Difol, were developed by Amoco Chemical Co. (now BP) several years ago[7]. These polybutadiene diols proved to be suitable components in the preparation of TPU elastomers[8]. However, Difol polyols have not been commercialized.
Krasol LBH polybutadiene diols, manufactured by Kaucuk and distributed exclusively through Sartomer and its sister company Cray Valley, are anionically polymerized products with very narrow molecular-weight distribution containing no species with functionality higher than 2.0[9]. Krasol LBH diols are available in the molecular-weight range from 2,000 to 10,000. The commercially manufactured products are terminated by secondary-OH groups; however, developmental grades of Krasol LBH-P with primary-OH groups are also available in limited amounts.
To estimate the potential use of Krasol polyols in TPU applications, the first phase of the study was focused on the development of TPU formulations. Special attention was given to the evaluation of the hydrolytic and chemical stability of the polybutadiene TPUs and to the measurements of their electrical-insulation properties. The data thus obtained were compared with those of polyether-based TPU Pellethane 2103-70A. This soft-grade TPU is recommended for applications requiring very good hydrolytic resistance and excellent electrical properties[10].
Experimental
Materials
All chemicals used in this study are listed in Table 1 and were used as received from the suppliers unless otherwise stated. Chain extenders were checked for the content of moisture and, if necessary, dried under a vacuum until the content of water was below 0.03 percent. Pellethane 2103-70A was measured using the thermomechanical analysis method to determine its softening temperature, which was 145 degrees C.
Preparation of Thermoplastic Polyurethanes
The synthesis of TPUs was performed in a one-liter glass reactor at normal pressure under a nitrogen blanket with vigorous agitation. The NCO/OH ratio of all formulations was 1.03-1.05. In the case of the prepolymer procedure, Krasol LBH diol was reacted with a diisocyanate at 80 degrees C for one hour to yield a prepolymer. The prepolymer was mixed in the second step with a chain extender at 100 degrees C for 10 minutes. The resulting material was poured into a mold and left to cure at 100 degrees C for 20 hours. Post-curing of the TPU proceeded at laboratory temperature for seven days. Under these conditions, the addition of a catalyst was not necessary.
In the case of the one-shot procedure, the reaction vessel was charged with Krasol LBH or LBH-P, chain extender, and Tinuvin B75, and the mixture was heated to 80 to 90 degrees C. Then, Suprasec MPR (preheated to about 45 degrees C) was added. After 3-5 minutes of agitation, the TPU was discharged into a mold and left to cure as described above.
The polyurethane sheets thus prepared were used for the determination of mechanical and physical properties and for the resistance study.
Extrusion of Thermoplastic Elastomers
Extrusion of the TPUs was carried out in a Goettfert extruder (Feinwerk Technik Buchen/Odenwald, Germany) with a screw diameter of 20 mm and three zones of electric heating adjusted to the temperatures 1/2/3=160/190/180 degrees C. The extruded strand was air-cooled and chopped into granules using a Scheer SGS-50-E machine.

Physical and mechanical properties of polyurethane elastomers were evaluated by the following test methods:
• Shore hardness (ISO 868:1985),
• Tensile stress-strain properties (ISO 37:1994),
• Compression set (ISO 815:1991), and
• Electrical properties, i.e., volume resistivity and surface resistivity (IEC 93), and electrometer Keithley 616.
The glass transition temperature (Tg) and the softening temperature values of TPUs were measured by thermomechanical analysis (TMA).
The resistance of polyurethane elastomers was studied using aqueous solutions of CH3COOH (10 percent), H2SO4 (60 percent), HNO3 (40 percent), NaOH (50 percent), water steam, methanol and ethanol. The temperature of the media was 23 degrees C except for water steam, which was 100 degrees C. The time of exposure was 28 days, after which the retention of Shore hardness was determined. The swelling of the polyurethanes in ethanol was monitored by measuring the volume change of the samples.

Results and Discussion
Thermoplastic Polyurethanes Prepared by Prepolymer ProcedureThe following parameters of the formulation were tested in order to find out the structure-property relationship of the Krasol-based TPUs:
• Type of OH groups (primary or secondary) of the polybutadiene diol and its molecular weight,
• Method of synthesis (one-shot or prepolymer procedure),
• Selection of the chain extender, and
• Hard-segment content.
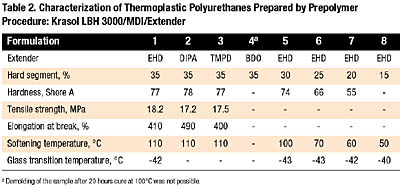
The first syntheses were performed using a secondary polyol Krasol LBH 3000 and adopting the prepolymer approach. The formulations numbered 1 through 4 in Table 2 show the comparison of 2-ethyl-1,3-hexanediol (EHD), 2,2,4-trimethyl pentane-1,3-diol (TMPD), N,N-diisopropanol aniline (DIPA), and 1,4-butanediol (BDO) as chain extenders. The use of EHD, DIPA or TMPD led to polyurethanes with Shore hardness of about 80 A, which may be classified as soft-grade TPUs. Their mechanical properties are comparable with those of good-quality, general-purpose rubber materials.
The use of BDO as an extender in Krasol-based TPUs with 35 percent hard-segment content cannot be recommended due to serious problems with demolding, which may be caused by the limited miscibility of polybutadiene diols with 1,4-butanediol (BDO)[8]. However, successful syntheses of TPUs based on hydrocarbon polyols have been reported when using BDO at relatively low hard-segment content (20-23 percent)[8,11]. This possibility for Krasol-based TPUs was verified later in one-shot formulations.
With regard to thermal characteristics, the melting point of hard segments formed by MDI and non-linear chain extenders is significantly lower than that of MDI/BDO domains[12]. Therefore, the softening temperature of TPUs in Table 2 is relatively low. The Tg, on the other hand, corresponds to the medium-vinyl-type polybutadiene in the elastomers’ soft segments.
The best results with good reproducibility were achieved with EHD and DIPA. The main difference between these two extenders is in processing since DIPA is a solid at ambient temperature. Based on these data, EHD was chosen for further development.
In an attempt to obtain even softer TPUs, a series of samples with decreasing hard-phase content was synthesized (Table 2, Formulations 5-8). By decreasing the hard-segment content, it was possible to prepare TPUs with hardness as low as 55 Shore A. However, a weak point of TPUs containing EHD as a chain extender and having 25 percent or lower hard-phase content is their poor heat resistance.
Preparations of the TPU Krasol LBH 3000/MDI/EHD with 35 percent hard-segment content (according to Table 2, Formulation 1) were carried out repeatedly to check the reproducibility of the synthesis. The typical characteristics of the elastomer were as follows: Shore A hardness 78-82, tensile strength 15-18 MPa, elongation 500-700 percent, compression set (70 degrees C, 22 hours) 50 percent, softening temperature about 110 degrees C, and Tg from -39 to -43 degrees C.
Some of the batches were prepared with the addition (1.0 percent) of Tinuvin B75, a complex polyurethane stabilizer. The presence of the stabilizer is expected to reduce light-induced yellowing of the polyurethane (due to the presence of aromatic isocyanate) and to minimize possible oxidation processes in the double bonds of the polybutadiene backbone. The addition of Tinuvin B75 did not affect the measured elastomer properties.
Thermoplastic Polyurethanes Prepared by One-Shot Procedure
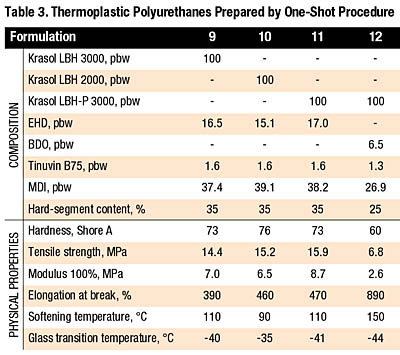
The Krasol LBH polybutadiene polyols, although having secondary-OH groups, can be used with EHD as a chain extender for one-shot TPUs. The effect of the synthetic procedure on the elastomer properties is shown by comparing Formulation 1 in Table 2 with Formulation 9 in Table 3. A one-shot TPU based on LBH 3000 exhibited somewhat lower hardness, as well as lower tensile properties, compared with the polyurethane prepared by the prepolymer method. Thermal characteristics of both products were equivalent.
The role of polyol molecular weight and of the type of OH groups can also be seen from Table 3 (Formulations 9 through 11). All of these elastomers have similar properties; however, the use of LBH 2000 resulted in a small increase of Shore hardness and a slight change in softening and glass transition temperature.
As mentioned earlier, only a limited amount of BDO can be added to the formulation. As a result, TPUs can be prepared only with hard-segment content not exceeding about 25 percent. The higher reactivity of BDO requires the use of the more reactive Krasol LBH-P in one-shot systems. A formulation with LBH-P 3000 and BDO yielded a very soft TPU product with hardness 60 Shore A, good elongation and relatively high softening temperature (Table 3, Formulation 12).

The processing possibilities were tested on TPUs composed of Krasol LBH 3000/MDI/EHD with 35 percent hard-segment content. The TPUs can be hot-pressed at 140-150 degrees C and extruded at 160-190 degrees C.
The melting and re-crystallization of the polyurethane hard segments were followed by changes in elastomer appearance. The TPU obtained after curing at 100 degrees C was white and not transparent. After hot pressing at 140-150 degrees C, the material became almost clear, whereas extrusion at temperatures of 180-190 degrees C yielded a transparent product.

Polybutadiene urethanes Number 1, 10 and 12 (cf. Table 2 and 3) and a conventional TPU based on poly(tetra-methylene ether) glycol (PTMEG) were included in the study.
The elastomers were tested for their resistance to aqueous solutions of a weak acid (10 percent CH3COOH), strong mineral acid (60 percent H2SO4), oxidizing mineral acid (40 percent HNO3) and strong alkaline solution (50 percent NaOH). All tests were performed at ambient temperature.
No significant differences were found among the Krasol-based TPUs with regard to their resistance to the individual media. Figure 1 compares the stability of the Krasol LBH-3000-based TPU with one having a polyether polyol as the soft segments. Both TPUs were stable in acetic acid and NaOH. However, a dramatic difference was found after soaking the elastomers in solutions of sulfuric acid and nitric acid. In these media, the polyether-based TPU corroded or dissolved after one day of immersion, while polybutadiene-urethane remained almost unaffected for 28 days.
Another series of measurements was carried out after immersion of elastomers in methanol and ethanol. Both polyether- and polybutadiene-based TPUs exhibited a considerable decrease in Shore hardness after 28-days immersion (Figure 1), which can be attributed to swelling. Remarkable differences between the TPUs were revealed by monitoring the volume change in the course of the test in ethanol (Figure 2). The polyether-based TPU increased its volume within one day by about 90 percent, and then remained without significant change until the end of exposure. The specimens withdrawn from alcohol after 28 days could be easily dried (24 hours at ambient temperature and normal pressure) to their original volume.
The polybutadiene-based material swelled slowly in ethanol, reaching the maximum volume change (approx. 20 percent) after about 14 days. By drying after the exposure, the solvent was removed only partially. These data suggest a very slow diffusion of polar molecules of alcohol through the hydrophobic matrix of polybutadiene polyurethane both into and out of the sample.
The study of the hydrolytic resistance of polyurethanes was extended to testing their stability to water steam at 100 degrees C (Table 4). Under these conditions, polybutadiene-urethanes with hard segments formed by MDI and EHD suffered from poor resistance upon heating. In this case, a polybutadiene TPU with MDI/BDO hard segments was used for the comparison since it has a similar softening temperature to that of the polyether elastomer. The data suggest that the resistance of Krasol and polyether-based TPUs to water steam is comparable.
Electrical Insulation Properties
Volume resistivity and surface resistivity were measured for polybutadiene-urethanes of various compositions (Table 5). The data obtained were compared with those of PTMEG-based TPU Pellethane 2103-70A, which is recommended for use in electrical-insulation applications. The value of volume resistivity found for Pellethane corresponded to the published data[10].
The detailed composition of polybutadiene-urethanes had no significant effect on the measured parameters. Polybutadiene TPUs exhibited superior electrical-insulation properties. Their values for volume resistivity and surface resistivity were higher by several orders of magnitude than those of the polyether TPU. The data indicate that polybutadiene-based TPUs could be desirable materials for wire and cable-jacketing or electrical-insulation and sealant applications.
Conclusions
Polybutadiene diols Krasol LBH (secondary-OH groups) and LBH-P (primary-OH groups) were used in the preparation of thermoplastic polyurethanes (TPUs) employing either a one-shot or a prepolymer procedure.2-Ethyl-1,3-hexanediol (EHD) was found to be a suitable chain extender for Krasol-based TPUs. 1,4-Butanediol can also be used in polybutadiene-urethanes provided that a hard-segment content of about 25 percent is not exceeded. Depending on the parameters of the formulation, the resulting TPUs exhibited Shore A hardness of 80 or lower, making them prospective materials by falling into the category of soft-grade TPUs.
The polybutadiene backbone provides an exceptional resistance against aqueous solutions of strong mineral acids (sulfuric acid, nitric acid) — far superior to that of polyether- or polyester-based elastomers. These TPU systems are ideal candidates for adhesive and sealant applications requiring resistance to aggressive protic media or the ability to ensure long service in humid conditions.
Acknowledgements
The authors wish to thank Hana Tajcova, Vera Pjatkanova and Sona Nemeckova at the Synthetic Rubber Research Institute of Kaucuk, a.s., for their contribution to the work described in this paper.References:
1. H. G. Hoppe and H. G. Wussow in Polyurethane Handbook, G. Oertel editor, 2nd edition, Hanser Publishers (1994), p. 421.2. S. Lee, Thermoplastic Polyurethane Markets in the EU: Production, Technology, Applications and Trends, Rapra Technology Limited, February 1998.
3. PolyBd, the Best of Two Worlds, Arco Technical. Bulletin, Arco Chemical Co., Div. Of Atlantic Richfield Co.
4. J. Pytela, M. Sufcak, J. Cermak and J. G. Drobny, “Novel Isocyanate Prepolymers Based on Polybutadiene Diols for Composite Binders and Cast Elastomers”, Proceedings of the Polyurethanes EXPO98, Dallas, September 1998, p. 563
5. J. Pytela and M. Sufcak, “Polybutadiene-Urethane Elastomers with Outstanding Resistance to Aggressive Aqueous Media”, UTECH 2000 Conference, The Hague, The Netherlands, March 2000, Conference Proceedings, Coatings, Adhesives, Sealants and Elastomers Session, Paper 9.
6. Poly bd Resins in Urethane Elastomers, Atochem Technical Bulletin, Atochem North America, October 1990.
7. H. B. Yokelson, P. O. Nubel, A. Sendijarevic, V. Sendijarevic and K. C. Frisch, “Novel Hydrocarbon-Based Diols for Polyurethane Elastomers”, Proceedings of the SPI Polyurethanes 1995 Conference, Chicago, September 1995.
8. K. C. Frisch, A. Sendijarevic, V. Sendijarevic, H. B. Yokelson and P. O. Nubel, “Polyurethane Elastomers Based Upon Novel Hydrocarbon-Based Diols”, Conference UTECH 96, The Hague, The Netherlands, March 1996, Book of Papers, Paper 42.
9. J. Pytela and M. Sufcak, “New Anionic Polybutadiene Diols for Polyurethane Systems”, Proceedings of the Polyurethanes World Congress 1997, Amsterdam, The Netherlands, September 1997, p. 704.
10. Products, Properties and Processing for Pellethane Thermoplastic Polyurethane Elastomers, Dow Plastics Technical Bulletin, The Dow Chemical Co., November 1997.
11. E. J. Gerard and J. K. L. Schneider, “Non-Polar Long Chain Hydroxyl Compounds Extending the Range for Polyurethanes in CASE and Foam Applications”, Proceedings of the Polyurethanes World Congress 1997, Amsterdam, The Netherlands, September 1997, p. 552.
12. W. Meckel, W. Goyert and W. Wieder in Thermoplastic Elastomers: A Comprehensive Review, N. R. Legge, G. Holden and H. E. Schroeder editors, Hanser Publishers (1987), p.21.

