A very good insight on polyurethane chemistry and technology is given in References 1-2; Reference 3 explores the chemistry of isocyanates. Additional publications not cited in this article can be traced in the relevant literature.
The global plant capacity of di- and poly- (methylene diphenyl isocyanates) is estimated at around 2.3 million tons, including new investments in the Far East. Major producers are BASF, Bayer, Dow and Huntsman.
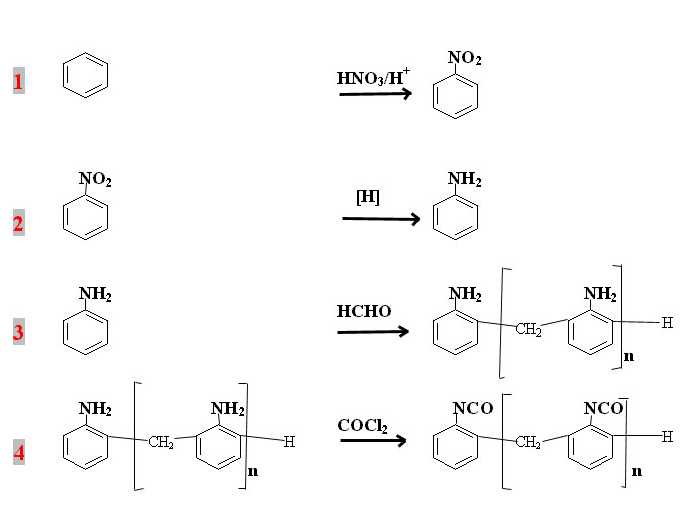
The phosgenation reaction, which is expressed in an ideal form in Step 4, has very often been a source of research programs aimed at the substitution of phosgene gas by easier-to-handle chemicals.
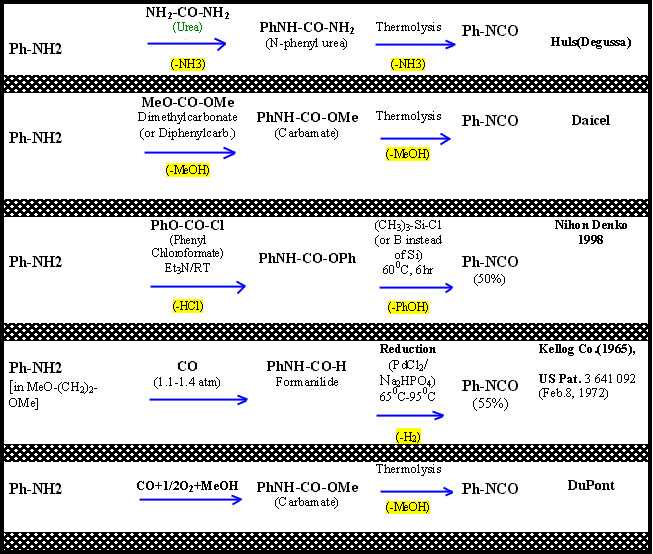
A few examples of amine (either aliphatic or aromatic) conversion into a mono-isocyanate through the use of phosgene alternatives, along with some references, are given in Figure 2.
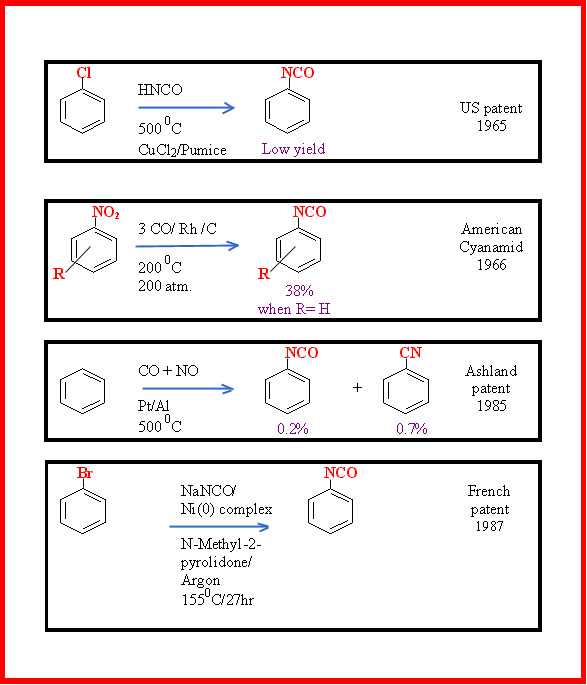
Other patented, synthetic approaches that avoid the passage through the amine are shown in Figure 3.
- The reduction of the steps involved in the current industrial production processes (Figure 1) - a welcome advantage considering the ever-increasing prices of the raw materials and the subsequent pressures they exercise on the profit margins of companies.
- Avoiding the hazardous phosgenation step, part of a continuing search for safer production methods.
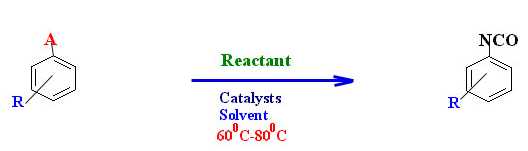
Such a synthetic route is represented in a simplified form in Figure 4.
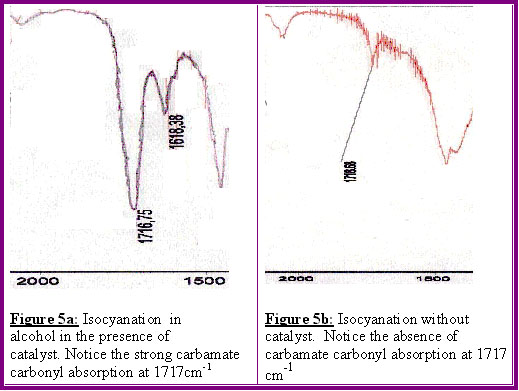
This is shown on the infrared spectra, sections of which are shown in Figures 5a-5b.
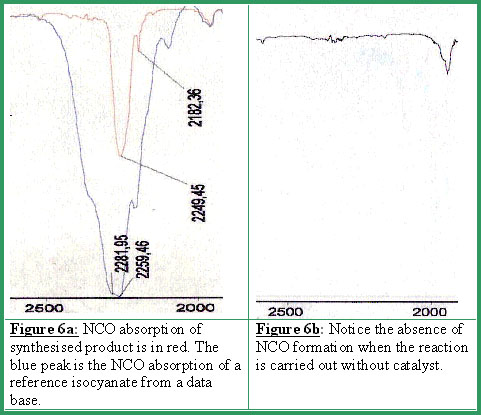
The latter is identified by the NCO group absorption at 2250 cm-1 (see Figures 6a-6b).
- Improvement of the reaction yield.
- Synthesis of methylene diphenyl isocyanate.
- Synthesis of linear aliphatic diisocyanates.
- Eventual scaleup of the process.
For more information, contact Demosthenes (Deny) Kyriacos, GEM-Chem, Avenue G. Mullie 25, 1200 Brussels- Belgium, phone 011-32-2-7710649; or e-mail GEM-Chem@skynet.be .