
Certain physical, chemical and mechanical characteristics render some materials more desirable than others for biological application. To complicate things further, the determination of these desirable traits depends on the material's intended use in the body. For example, the material for a bone implant must have greater compressive strength, while the material used for a ligament replacement must display far more flexibility and tensile strength. However, in all cases, the biomaterial must be compatible with the human body.
A number of adhesives and sealants are suitable for short- and/or long-term biomedical applications. Typical adhesives used in medical products include silicones, epoxies, acrylics and polyurethanes. Perhaps the most interesting materials, however, are those that are aimed at surgical applications.
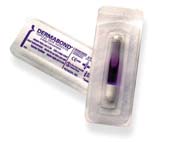
Today, most of these are based on cyanoacrylate materials. Two such commercial products are Dermabond, surgical glue that replaces suture and staples, and Liquid Band-Aid, a protective covering for cuts and scrapes that acts as a bandage. Both of these products were developed by Closure Medical Corp., a small start-up company located in Raleigh, NC. Johnson and Johnson, one of the world's largest manufacturers of heath care products, has recently agreed to acquire Closure.
The application of hydrogels covers a broad range. Hydrogels can control the release of drugs by swelling and dehydrating depending on composition and environment. Soft contact lenses also employ hydrogel materials to allow gas exchange and to induce moisture equilibrium between the lens and the surface of the eye. Hydrogels are also being developed as artificial cartilage and skin.
Biomaterials are not only being used as adhesives for the human body-they are also being developed as sealants. In addition to the two products mentioned above, Closure is developing several surgical sealants. Omnex, a vascular sealant is about to become available in Europe. This sealant, the first surgical glue for use inside the body, can be used to glue blood vessels. A sealant for use after lung cancer surgery is at least two years from commercialization, and an orthopedic sealant for tendon and muscle repair is at least three years from market.
In the future, biomaterials will not only be compatible with the human body, but they may actually be bioactive. While biocompatible materials affect the equilibrium of the body as little as possible, bioactive materials encourage specific reactions between the material and the surrounding tissue. One example of such a bioactive material is hydroxyapatite, a polymer used as a porous coating on hip implants. The coating encourages growth of surrounding tissues that interlock into pores to help stabilize the stem of the implant in the bone.
One day, adhesives and sealants will not just be all around us-they may be inside us as well.
For more information, visit www.specialchem.com.
