
Table. Base Polymers Made and General Physical Properties
This article provides an introduction to the new technology and addresses how to achieve two-part performance in a one-part package.

Figure 1. Aluminum Tris(acetylacetonate) and Titanium Dialkoxide Bis(acetylacetonate)
Chemistry
In general, acrylic PSAs must be crosslinked to improve creep and shear resistance. Though this can be accomplished in many ways, this discussion will address one-part metal chelate chemistry and two-part reactive chemistry.One-part metal chelate systems use aluminum or titanium (see Figure 1) to crosslink between carboxylic acid groups on the polymer. This mixture gives reasonable in drum stability (3-12+ months), is easy to formulate and gives acceptable heat resistance for many applications.
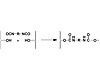
Figure 2. Example of Hydroxyl Functional PSA Reacting with a Diisocyanate
The advantage of a two-part system is that the ensuing product has covalent crosslinks. This results in higher cohesion at elevated temperatures compared to typical one-part metal chelate crosslinking systems. Disadvantages include short pot life and potential batch-to-batch variability. In addition, without a meter-mix-dispense system at the coating head, drums are mixed one at a time just prior to coating.

Figure 3. Acid and Epoxy Reaction
Henkel has developed a method for polymerizing acrylic or methacrylic acids in the presence of high levels of glycidyl acrylate or methacrylate. Crosslinking occurs upon coating and drying, not during manufacture or storage.
Experimental
Several base polymers of various Tgs and molecular weights were prepared using standard free-radical polymerization methods.Formulations
The flexibility of the base polymer allows for several formulation options. Base polymers were either used as is, with additional crosslinker, or with additional crosslinker and tackifier.Dynamic viscoelastic properties were determined using an ARES-M from TA Instruments in a parallel plate mode using 8-mm diameter plates. The temperature was scanned at 5°C/min. at a constant frequency of 10 rads/sec. with no more than 30% strain.
Samples were either transfer coated from release liner or direct coated onto the polyester film. The coating weight varied from 25g/m2to 90g/m2. The tape samples were stored at 21°C and 50% humidity for a minimum of 16 hours prior to testing.
Tape tests were done according to PSTC test methods on stainless-steel panels. In addition, aluminum, HDPE, polypropylene and painted panels were used for selective testing. The painted panels were obtained from ACT test panels and represent topcoat chemistries from BASF, DuPont, and PPG.
Results and Discussion
For comparison purposes, five different base polymers were made. These polymers varied by composition (Tg) and molecular weight, and are listed in the table.Samples A and B are very similar in composition but are adjusted for Tgand molecular weight. Samples D and E are very similar in composition but are adjusted for Tgand molecular weight. Sample C is a unique composition but is the lowest molecular weight of the group.

Figure 4. Peel of a High-Performance Acrylic vs. Base Polymer A on Various Substrates
PSA Performance Data
Figures 4-7 show representative performance data of base polymers A, B and C compared to a standard, high-performance metal chelate system, and for a tackifed system vs. a standard tackified acrylic.The standard tape grade uses aluminum metal chelate chemistry for crosslinking, and the new grade has no additional crosslinker. Both grades have excellent 70°C hot shear (>10,000 min.; 2.2 psi). All peel failure modes were adhesive failures, and shear failures were cohesive.

Figure 5. Peel of a Standard Tackified Grade vs. Formulated Polymers B and C
Tackification
Tackification is commonly performed to increase peel and tack, and to improve adhesion to plastics such as HDPE. Figure 5 shows how two of the new polymers, once formulated, compared with a standard tackified acrylic.
Figure 6. Elevated Temperature Cohesive Strength Comparison

Figure 7. Commercial Grades vs. R&D Grade on Painted Panels
Painted Panel
We obtained panels with clearcoats from BASF, DuPont, and PPG, and covered a range of solvent- and water-based chemistry. Early work indicated that these adhesives performed well on some current-generation painted panels.Figure 7 shows a marked improvement over commercial grades that were previously successful in this area. The mechanism for improved adhesion has not been fully explained, but is believed to be specific adhesion dominated.

Figure 8. Typical Rheological Profile of Base Polymers vs. Formulated Adhesive
Rheology
A typical rheological profile is shown in Figure 8. This figure compares base polymer C with a formulated version. The figure shows the expected increase in Tgand decrease in modulus achieved with tackification. The formulated adhesive contains a low level of extra crosslinker, which shallows the cure (that is, the change in slope of the modulus is less steep vs. unformulated). Note the significant cure that occurs at about 125°C (257°F); this is a result of the acrylic acid/glycidyl methacrylate reaction. By changing composition, process and formulation, we can manipulate the glass-transition temperature, Dahlquist criteria temperature, and modulus.
Figure 9. Comparison of Polymers D and E
Conclusion
The chemistry discussed in this article offers the extended shelf life of a one-part system with many of the advantages of two-part crosslinking. These polymers have shown a range of Tg, solubility parameter and functional-group tolerance. In addition, these adhesives have demonstrated particularly good adhesion to the new generation of automotive topcoats.We believe that the unique blend of molecular weight, crosslinking, and formulation potential afforded by this approach can offer a strong balance of properties - maintaining cohesive strength while achieving high levels of peel adhesion and tack.
Acknowledgements
The author would like to acknowledge the entire project team, with special recognition to Martijn Verhagen for his polymerization expertise and guidance. In addition, thanks are given to Omar Anwary, Bruce Stevens and Karen Freeman for their assistance with performance testing.For more information on acrylic PSAs, visit www.henkel.com.
This article is based on a paper presented at the Pressure Sensitive Tape Council Week of Learning. For more information, visit www.pstc.org.