Significant advances have been made over the past 20 years in the development of biodegradable polymers. These materials have been developed in a variety of forms, and thus have potential uses in a range of industries. Many of these polymers are suited for adhesive applications, such as environmentally friendly packaging, recyclable envelope adhesives and biomedical devices.
Biodegradable polymers based on renewable resources are also being seen as low-cost alternatives to petroleum-based raw materials. Industries that develop these materials will continue to see growth, as the price of crude oil keeps climbing and the availability of fossil fuels begins to dwindle. Applications for such bio-based materials are widespread in the areas of resins, coatings and adhesives.
The development of biodegradable adhesives goes hand-in-hand with the development of biodegradable plastic products. In order for the finished product to be completely biodegradable, all of its components must be biodegradable. A range of potential biodegradable products is available, including the following:
- Packaging materials (trash bags, loose fill foam, food containers)
- Consumer goods (egg cartons, razor handles, toys, straws, utensils)
- Industrial products (planter boxes, compositing bags, fishing nets, mulch film)
- Medical applications (drug-delivery systems, sutures, bandages, orthopedic implants)
- Coatings (barrier coatings with low moisture vapor transmission of paper and plastic film)
- Hygiene products (flushable sanitary products, diaper backing sheets)
Many of the polymers developed specifically for these products may also be suitable base polymers for biodegradable adhesive systems.
This article identifies the key issues for biodegradable adhesives, including the following:
- The market potential and growth opportunities for biodegradable polymers
- The various types and composition of biodegradable polymers that may be appropriate for adhesive formulations
- Recent commercial activity in this area
Definition of "Biodegradable"
The failure of early biodegradable polymers to properly degrade has led the ASTM to create a definition for what constitutes “biodegradability.” Biodegradability means that a product is “capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass in which the predominant mechanism is the enzymatic action of microorganisms that can be measured by standardized tests, in a specific period of time, reflecting available disposal conditions”.1 Many so-called biodegradable polymers are actually bioerodable, hydro-biodegradable or photo-biodegradable.
Hydro-biodegradable and photo-biodegradable products are degraded or broken down in a two-step process: 1.) Hydrolysis or photo-degradation; and 2.) Further biodegradation as defined above by the ASTM. Products can also break down by a single-phase process (water soluble or photo-degradation) where the residue is not further degraded by organisms. Bioerodable polymers are capable of degrading without the action of microorganisms, at least initially. Their degradation processes may include dissolution in water, oxidative embrittlement or UV embrittlement.
All of these polymers come under the broader category of “environmentally degradable” polymers. For the purpose of this article, the term “biodegradable” shall also imply “environmentally degradable.”
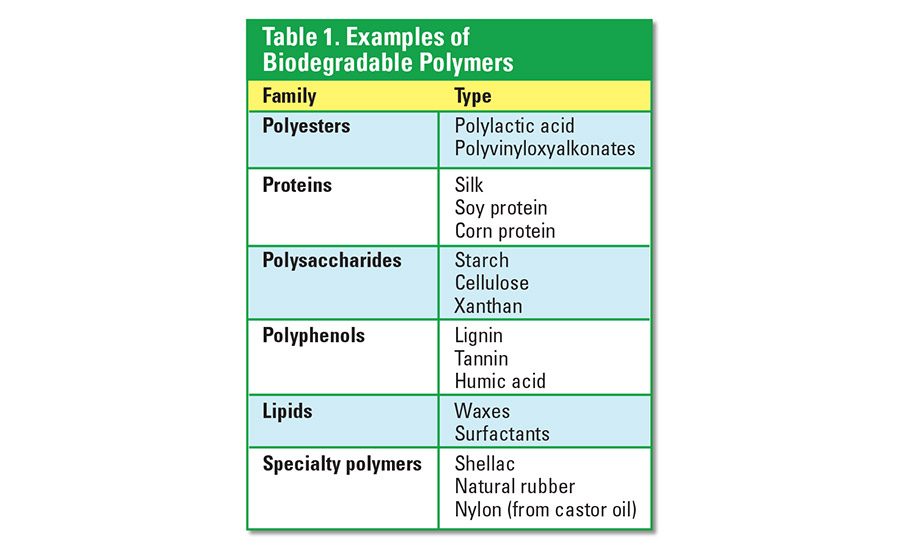
Most synthetic polymers are not biodegradable. Polymers such as polyethylene and polypropylene can exist in the environment for many years after their disposal. Biodegradable polymers are generally obtained by way of polymerization of bio-based raw materials. These raw materials are either isolated from plants and animals or synthesized through modern industrial processes. Examples of biodegradable polymers are provided in Table 1.
Market Potential of Biodegradable Polymers
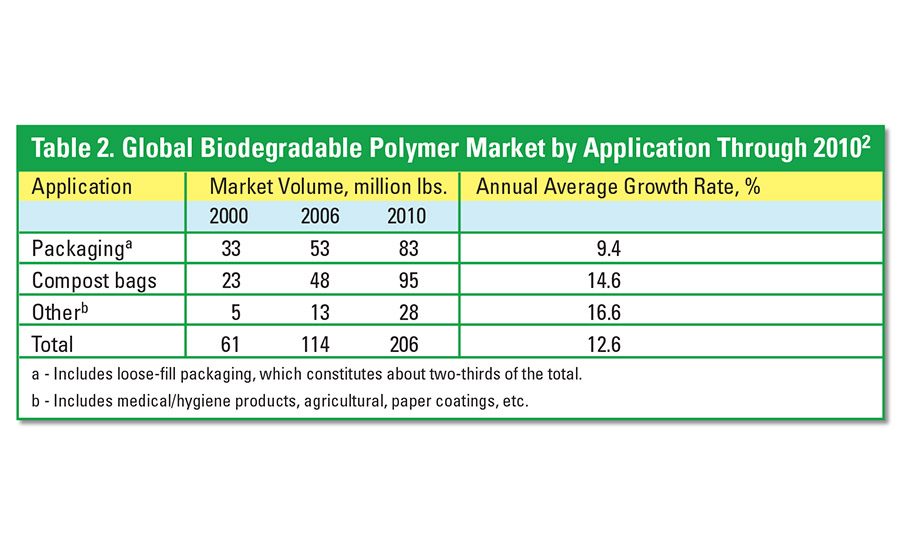
The global biodegradable polymers market is currently estimated at 114 million lbs.2 Average annual growth rates are far in excess of the GDP, with forecasts for the market to be well over 200 million lbs by the end of the decade (see Table 2).
Packaging, which includes loose-fill packaging, made up nearly 47% of the total biodegradable polymers market in 2005. However, compost packaging will represent nearly 50% of the market by 2010. Other important products, such as medical/hygiene, agricultural, and paper coatings, accounted for 11% of the total applications in 2005.
The North American biodegradable polymer market has not progressed as rapidly as it has in Europe and Japan. The major drivers for the U.S. market are mandated by legislation and prospective increases in landfill cost. For significant growth in North America, systems or infrastructures must first be installed to collect and process biodegradable polymers, consumers must be willing to accept the inconvenience and cost, and biodegradable products must be viewed as a realistic and available alternative to waste disposal by all parties.
Starch-Based Biodegradable Polymers
Starch is a pure, natural biopolymer found in the roots, seeds and stems of plants such as corn, wheat, and potatoes. It is suitable for chemical modification into a thermoplastic material for various applications. Starch is fully biodegradable and based on renewable materials. Thus, its use in commercial adhesive compounds and in plastic materials will minimize environmental damage.
Starch degrades by molecular breakdown resulting from enzymatic attack on the glucosidic linkages between sugar groups. These products have starch contents that vary significantly. The starch content needs to exceed 60% for significant material breakdown, although most starch-based biodegradable polymers have starch contents of 10-90+%. As the starch content is increased, the polymers become more biodegradable. At low starch contents, the starch particles act as weak links in the polymer matrix and provide sites for biological attack.
Starch could be the original biodegradable adhesive. It plays a very large part in industrial production, especially the packaging industry. Starch-based adhesives are principally used for bonding paper products and other porous substrates. Most corrugated boxboard for making cartons is easily bonded with starch-based adhesives.
There are many benefits to starch adhesives. They are readily available, inexpensive and easy to apply through water dispersion. They are considered to be the least-expensive class of paper-packaging adhesive. Formulated starch adhesives can be applied hot or cold. The adhesives are generally provided to the end user as powder and mixed with water prior to use to form a relatively thick paste. Starch and dextrin cure through the loss of moisture; since these adhesives cure to a thermosetting structure, they have excellent heat resistance. Another advantage is their very slow curing rate, allowing ample assembly time. Disadvantages include poor moisture resistance and mold growth.
Although starch-based adhesives have been used for many decades, there are several important reasons why these natural adhesives will not be entirely replaced by synthetic products. The following advantages ensure that they continue to fill particular niches in the marketplace.3
- Availability is good and cost is relatively low
- Quality is stable
- Good adhesion to cellulose and many porous substrates
- Insolubility in oils and fats
- Non-toxic and biodegradable
- Heat resistance
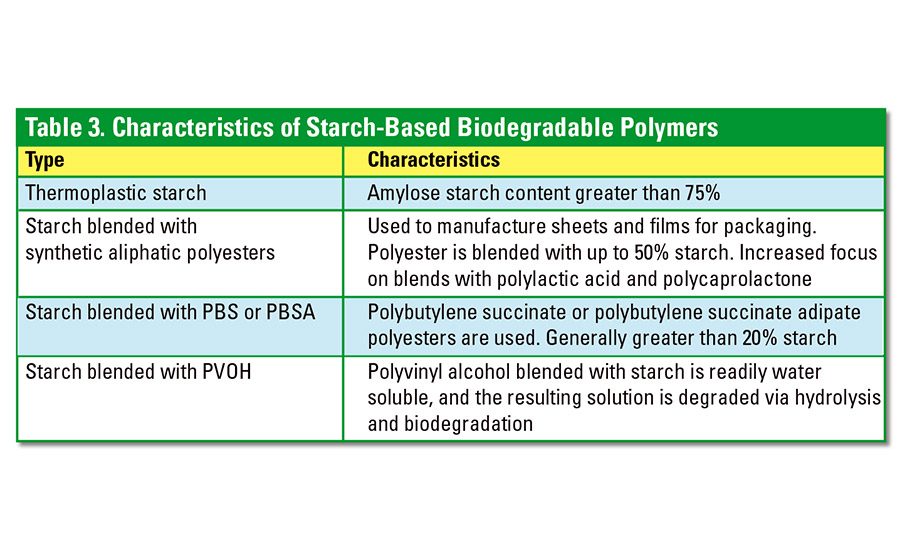
To meet the requirements of various modern applications, starch-based polymers may be blended with higher-performance biodegradable polymers, such as aliphatic polyesters and polyvinyl alcohols. Adding starch to a polymer mix reduces the volume of synthetic materials required, thus reducing the overall material cost. Varieties of biodegradable starch-based polymers include thermoplastic starch, starch synthetic aliphatic polymer blends, starch-polybutylene succinate (PBS)/polybutylene succinate adipate (PBSA) polyester blends and starch-ethylene vinyl alcohol (EVOH) or polyvinyl alcohol (PVOH) blends. The characteristics of these materials are summarized in Table 3.
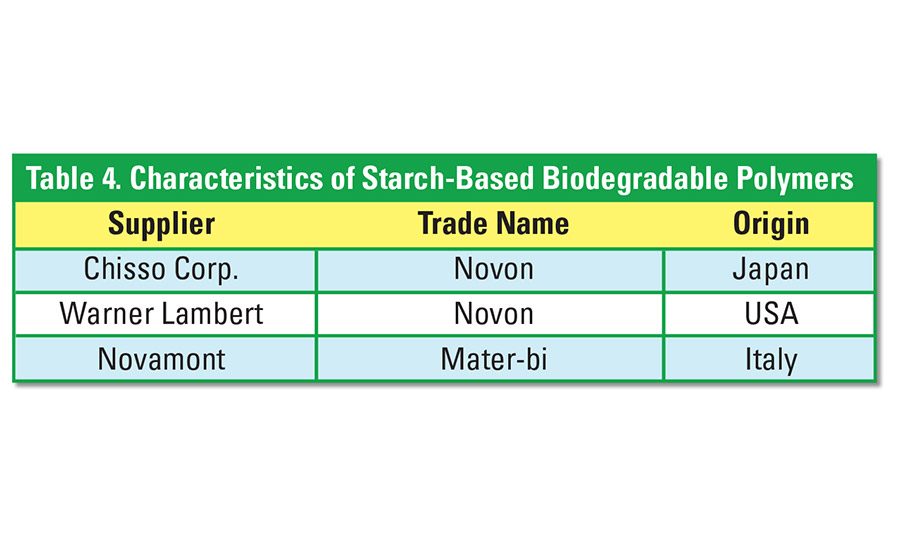
Polyvinyl alcohol (PVOH) is blended with starch to produce readily biodegradable polymers. The PVOH is very water soluble, and the starch-PVOH blends are, therefore, degraded by way of hydrolysis and biodegradation of the sugar molecules. Table 4 identifies some of the starch-PVOH blends that are commercially available.
As starch is fully biodegradable and easily renewable, it will continue to be an important component of the biopolymer industry.
Other Plant-Based Raw Materials
Several other biologically based raw materials have been used in adhesive systems. Since the beginning of time, plants have been known to act as feedstock for a variety of chemical applications. As of late, there has been a return to agriculturally based chemicals. The development in this area is primarily due to environmental regulations and conservation, but biodegradation of waste has contributed as well. In addition to starch, much work has been done on adhesives based on casein, a material derived from milk.
Linseed oil is a common feedstock for resins and coatings. It can be used to derive several high-performance polymer resins, including polyester amide. Hemp oil-based products are developed for exterior coatings. The material boasts high environmental awareness and is derived from a renewable resource.
Castor oil and soybean-based polyols are examples of plant materials that have been successfully incorporated into polyurethane resins. Epoxidized oils are synthesized by reacting vegetable oils (typically soybean and linseed) with peracids or hydrogen peroxide. These epoxidized oils are used as biodegradable plasticizers for adhesives and plastics. Long-chain fatty acid dimmers derived from vegetable oils are reacted with a slight excess of primary amines to synthesize polyamides, which are commonly used as curing agents in epoxy coatings and adhesives.
Biodegradable Polyesters
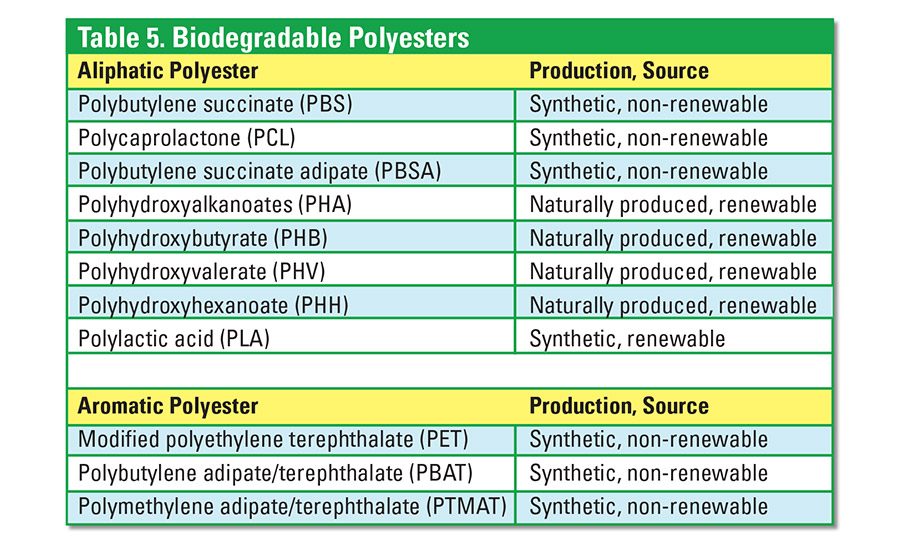
The potentially hydrolysable ester bonds of polyesters make them significant raw materials in the development of biodegradable polymers. The polyester family is made of two major groups: aliphatic (linear) polyester and aromatic (ring structure) polyesters. Biodegradable polyesters that have been developed commercially and that are in the development stages are shown in Table 5.
All polyesters degrade eventually, with hydrolysis being the dominant mechanism. Aliphatic polyesters are commonly used because they are more readily biodegradable than aromatic polyesters. Synthetic aliphatic polyesters are easily biodegradable in soil. These aliphatic polyesters are, however, more expensive and lack mechanical strength when compared to conventional plastics. Aliphatic polyesters are frequently combined with starch to reduce material cost. Compositions are blended with nanoclay reinforcement to improve mechanical properties and barrier performance in food-packaging applications. Polylactic acid (PLA) is the most widely used biodegradable polyester.
Lactic acid is produced principally by way of microbial fermenting sugar feedstock. Making PLA requires 30-50% less fossil fuel than polymers synthesized from hydrocarbons. Variation in polymerization conditions permits the synthesis of several grades of PLA. Polylactic acid degrades primarily by hydrolysis and not through microbial attack. It does not biodegrade readily at temperatures lower than 60°C due to its glass-transition temperature of close to 60°C.
PLA has high polarity, making it difficult to adhere. Tie layers generally must be used to provide a bond to non-polar PE and PP in multi-layer structures. PLA has excellent heat-sealing performance. PLA-based materials, such as those produced by NatureWorks LLC under the trade name of NatureWorks, are most commonly used in packaging as thermoformed products, such as drinking cups, takeout food trays and other containers.
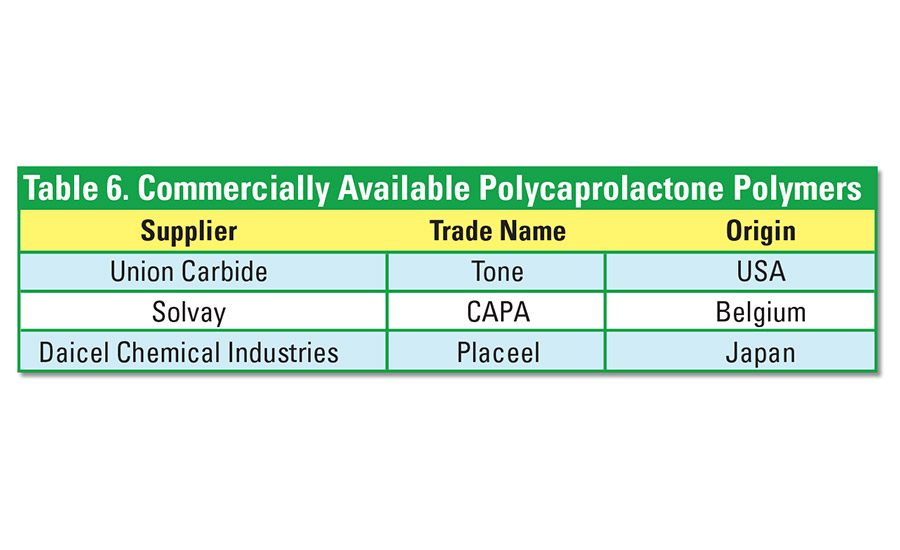
Polycaprolactone (PCL) polyesters are low-viscosity synthetic aliphatic polyesters. Their relatively high cost can be overcome somewhat by the addition of starch. Derived from non-renewable sources, PCL is fully biodegradable through a single process of composting at 60°C. Without additives, it completely biodegrades after about six weeks in compost activated with sludge. Processing additives increase tensile strength but lower biodegradability. See Table 6 for a list of companies that supply PCL materials.
Soybean-Based Adhesives
Soybean flour is formulated into adhesive systems as the main polymeric component, incorporated as extenders for phenolic resins, or blended with casein or sodium-silicate adhesives. Soybean glue is derived from protein. These adhesives are cheap and can be used for making semi-water-resistant plywood, and for coating some types of paper. They are primarily used to manufacture plywood, and set at room temperature.
One-package soybean glue formulations are dry powders. Soybean powder contains both protein and carbohydrate. For adhesives, the flour is generally dispersed in aqueous sodium hydroxide, and other alkaline bivalent metallic ions - such as calcium hydroxide - are incorporated to lengthen working time and improve water resistance.
Soybean glues are generally considered to have limited water resistance, but, like casein glues, recover their strength on drying. They are susceptible to mold growth, but a range of preservatives is available, such as pentachlorophenol, copper naphthenate and tributyl tin oxide. Fungicides also provide some degree of termite protection.
Many polyfunctional materials are used as crosslinking agents for soybean proteins. Typical denaturants and crosslinking agents include sulfur compounds, such as carbon disulfide; soluble metal salts; epoxies; and formaldehyde donors, such as dimethylurea or trimethylphenol. Small proportions (usually under 1% of the dry weight of soybean flour) are sufficient. Fillers, such as wood flour, walnut-shell flour and clay, reduce cost, but they also lower the adhesive’s performance properties.
Soybean glues, like blood glues, can be safely used in the cold for wood lamination. However, for rapid production of assemblies, where several cure cycles are performed per hour, it is necessary to use temperatures as high as 140°C with pressures of 175-200 psi applied to the joint.
Modern soybean adhesives are made by combining hydrolyzed soy protein with phenol resorcinol formaldehyde. Compared to standard phenol resorcinol formaldehyde adhesives, this hybrid adhesive has a faster cure time and could bond wood with moisture content as high as 150%. Thus, soy-based adhesives can join green lumber, which allows lumber mills to operate with less energy and fewer harmful emissions. New research at Iowa State University has resulted in formulations that can replace up to 70% of the phenol formaldehyde in adhesives used to bond a variety of wood and fiber-based composite products.
Other Biodegradable Polymers
Water Soluble Polymers
There are two primary water-soluble polymer types: polyvinyl alcohol (PVOH) and ethylene vinyl alcohol (EVOH). PVOH is readily biodegradable and water soluble. It is commonly used in many conventional adhesive systems. It is often blended with polyvinyl acetate (PVAc) for wood adhesives. The degradation of PVOH is influenced by its crystallinity and molecular weight. The main degradation mechanism is dissolution in water. Biodegradation in soil is very slow.
EVOH is another water-soluble synthetic polymer, often used as an oxygen barrier layer in multilayer film packaging. It can be used as an effective tie-layer in laminating multilayer product. The high cost of EVOH is a significant barrier to its widespread use in biodegradable plastics.
Photo-Biodegradable Plastics
Photo-degradable polymers are polymers into which light-sensitive chemical additives or copolymers have been incorporated in order to weaken the bonds of the polymer in the presence of UV radiation. Photodegradable polymers are designed to become weak and brittle when exposed to sunlight for prolonged periods. Photosensitizers used included diketones, ferrocene derivatives and carbonyl-containing species. These plastics degrade in a two-stage process, with UV light initially breaking the molecular bonds.
Controlled Degradation Through Additive Masterbatches
Additives that impart controlled degradation behavior to conventional thermoplastics, as well as to inherently biodegradable polymers, are becoming a popular strategy due to price competition. Such additives are known as prodegradant concentrates, and are generally based on catalytic transition metal compounds, such as cobalt stearate or manganese stearate. The additive is typically used at levels of 1-3% and, therefore, may be a practical solution for many biodegradable adhesive formulators.
The principal company that has developed these prodegradant additives is EPI Environmental Technologies, Conroe, TX. Their products are trade named TDPA, or Totally Degradable Plastic Additives. Polymers modified with TDPA progressively degrade to lower and lower molecular weights. They become brittle, disintegrate and are ultimately digested by microorganisms.
Recent Commercial Activity in Biodegradable Adhesives
There are only a few major players in the global biodegradable polymer business, including NatureWorks LLC in North America and Novamont and BASF in Europe. Many Japanese companies are involved, but they have relatively small production volumes, including some pilot plant operations. There are far less companies active in developing biodegradable adhesives. Following is a summary of recent activities in this area.
Bio-based raw materials are now finding their way into adhesives applications where recycling and environmental concerns are important. Examples include a family of harmless, reactive, sugar-based monomers called ECOMER (EcoSynthetix Inc., Lansing, MI). Copolymerization of these monomers with acrylic monomers has resulted in sugar-based acrylic pressure-sensitive adhesives for recycling. This adhesive is being used in environmentally benign, self-adhesive postage stamps.
PSAs are also being developed at the University of Delaware from plant oil derivatives, such as fatty acid methyl esters. The rheological properties of the resulting polymers are comparable to petroleum-based polymers used in pressure-sensitive adhesives.
Natural-based adhesives have also been formulated from components in the forestry industry. A number of countries have made efforts to extract adhesive for particleboard manufacture from the barks of their indigenous trees. These woods generally have a high tannin content. This eliminates the need for relatively expensive phenol resins. Special formulations have been prepared with the combinations of tannins with melamine formaldehyde, phenol formaldehyde and isocyanates. Adhesives using sulphite waste liquor from the forest industry have also been developed as a particleboard adhesive.
The most common form of rosin adhesive is made from the oleoresin of the pine tree. This material is used either in solvent solution or as a hot-melt mastic. It has poor resistance to water and oxidation. Bond strengths are moderate and develop rapidly; thus this adhesive is often used for temporary fastening.
One of the most tenacious bio-adhesives in existence is that produced by the common sea mussel. The mussel depends on its ability to attach to solid surfaces for its survival. Poor adhesion for a mussel means that it can be dislodged from its marine home (generally wet rock, wood, or the steel hull of ships) and crushed by waves or lost to some other nautical fate.
Chemists have long known that mussels secrete a material that consists of a hardened matrix of proteins. These proteins form extremely tough fibers that adhere to almost anything, and under almost all conditions. Exactly how these proteins link together to provide an adhesive material, called byssus, has remained a mystery until recently.
Researchers at the University of California-Santa Barbara have studied the mussel and concluded that the secret to the mussel glue’s stickiness seems to be 3,4-dihydroxyphenylalamine (DOPA). DOPA is an amino acid found abundantly in the mussel’s bonding proteins.4 Further research at Purdue University indicates that another key ingredient in the mussel adhesive is iron.5,6 The mussel obtains iron by filtering it directly from its surrounding water. It uses iron as a “crosslinker.” A single iron atom can bind to three DOPA side chains. Other bio-available metal ions do not appear to bring about this crosslinking.
Unfortunately, the amount of adhesive protein that can be directly gained from marine organisms, such as the mussel, is quite small and expensive to harvest. Thus, we don’t believe that adhesive manufacturers will replace their reaction vessels with mussel farms. However, the biosynthesis of these marine-adhesive proteins can be copied, and production methods are possible and under development.7
It is believed that adhesives having molecular structure similar to the mussel secretions will be biodegradable and provide strong and durable structures. One goal of trying to mimic mussel adhesives is the preparation of better marine adhesives that can cure underwater, stick to almost any substrate and last a lifetime. However, the value may be in another water-rich application - medical adhesives. The ability of the material to bond to nearly any surface - not to mention its biological origin - make it well suited for many internal medical applications. Man-made mussel adhesives could be used for wound closure, nerve reconstruction, or when one might need a scaffold upon which to grow cells and build new tissue.8
References
- ASTM Standard D 5488, American Society of Testing and Materials, 1994.
- RP-175R Biodegradable Polymers, Business Communications Co. Inc., 2006.
- Lazarus, D.M., “Adhesives Based on Starch,” Chapter 10, Adhesives and Adhesion, Vol. 7, K.W. Allen, ed., Applied Science, London, 1983.
- Kintisch, E., “Sticky Science: Adhesives and Glues,” Chemistry, Winter 2004, pp. 30-34.
- “Marine Superglue,” Science News, Vol. 165, No. 3, January 17, 2004.
- “Sticky Science,” Adhesives & Sealants Industry, May 2005, p. 24.
- Quack, R., “Proteins-Innovative Base Materials for Adhesives and Coatings,” Chemical Business, June-July 2002.
- “Sticky Science,” Adhesives & Sealants Industry, May 2005, p. 24.