Isocyanates react with compounds that have active hydrogens. For example, in coating applications, isocyanates are typically reacted with polyols to form polyurethanes, or with primary and secondary amines to form polyureas. Catalysis of the polyol/isocyanate reaction, which is often required to develop sufficient cure response, can be achieved with a variety of compounds. The most common catalysts for the polyol/isocyanate reactons include metal salts, organometallic compounds and tertiary amines. Generally speaking, bases, acids and even water can, in some cases, accelerate isocyanate reactions.
Catalyst selection can be a very complicated process, particularly for urethane coating formulations, which contain ingredients that can either accelerate or decelerate the reaction rate by interacting with catalysts or active resins. Among the most common catalyst deactivating components in typical polyurethane formulations are water (hydrolysis), pigment (absorption), solvents (solubility) and acid groups (formation of inactive salts).
Of these components, water is a particular concern in polyurethane systems because it can deactivate catalysts by hydrolysis, and can react with free isocyanate to eventually form polyurea with a carbon dioxide (CO2) byproduct (gassing). Along with affecting the rate of reaction, different catalysts can provide reactions with distinctive profiles. Reaction profiles are particularly important when the product is cast into molds. Dibutyltin dilaurate (DBTDL) is often used in coatings applications; mercury catalysts can also provide desirable characteristics in many non-coating casted mold applications. Both are becoming increasingly regulated.
Since the early 1980s, when countries started placing restrictions on tributyltin and prohibiting the use of tributyltin in anti-fouling marine paint, the human health risks and the environmental issues associated with tin compounds have been increasingly scrutinized. Dibutyltin is the hydrolysis product of tributyltin antifouling compounds. Dibutyltin compounds can potentially contain trace levels of tributyltin. Organotin compounds are considered to be persistent organic pollutants and are regulated by the International Maritime Organization's Antifouling System (IMO AFS) convention and the U.S. Organotin Antifouling Paint Control Act (OAPCA). In addition, current REACH legislation restricts the use of organotin compounds in Europe; the European Commission Decision 2009/425/EC claims organostannic compounds in consumer products pose a risk to human health. This will increase the scope of restrictions beyond maritime markets.
The U.S. Environmental Protection Agency (EPA) considers mercury to be a persistent, bioaccumulative and toxic (PBT) pollutant. Mercury and other toxic heavy metals that are embedded in cured media, such as elastomers, sealants and coatings, can become health risks when they are extracted by acid rain and washed into groundwater.
This article demonstrates the performance of a variety of coating and non-coating systems catalyzed with unique and versatile catalysts that do not contain any tin or mercury. These catalysts can meet the efficiency standards of DBTDL and mercury while avoiding the environmental issues associated with DBTDL and mercury.1
Coatings
When formulating coatings, an efficient and versatile catalyst is needed that is selective to the polyol/isocyanate reaction while avoiding many of the traps associated with complex formulations that contain potential deactivating components. Formulators would generally prefer to work with a "universal" catalyst (i.e., one that can work in all urethane systems). Although DBTDL is not a very selective catalyst, it is considered to be relatively versatile and efficient for many polyurethane coating applications. Environmental and performance issues, however, have driven formulators to search for tin alternatives, preferably alternatives that are as versatile and efficient as DBTDL. Presently no "universal" catalyst can satisfy all requirements in all coating formulations.
Catalyst Selectivity
Catalysis of an isocyanate reaction to produce urethane is a selective process. Isocyanate groups (R-NCO) react with compounds containing active hydrogen atoms, such as alcohols, phenols, amines, carboxyl groups and water. The reaction of the hydroxyl-containing polyol with isocyanate is the desired reaction leading to urethane linkage in a coating.
Therefore, a catalyst that would selectively enhance this reaction would be desirable. Catalysts that are less selective can accelerate side reactions with other active hydrogen-donating components. A particular concern is the reaction of isocyanate with water. The eventual product of an isocyanate/water reaction is polyurea with an intermediate reaction, which produces a primary amine and releases CO2.
This side reaction can be detrimental to the quality of a formulated coating. Sufficient generation of CO2 causes gassing, which reduces the time that the paint is usable and causes gloss reduction in applied films. In solvent-based systems, water can be carried into a formula by pigments, resins, solvents and other additives, and can be present as atmospheric humidity. Naturally, catalyst selectivity in water-reducible systems is a major concern.
Recent studies suggest that certain zirconium complexes are more selective catalysts than DBTDL. Based on this work, specific zirconium catalysts have been developed that can provide fast dry times by selective catalysis of two-component acrylic and polyester urethane coatings crosslinked with HDI trimers and biurets.2 In two-component waterborne systems, these zirconium catalysts must be incorporated on the isocyanate side, which is sometimes impossible.
A more efficient and versatile alternative to tin-based catalysts has been developed that can overcome several of the issues associated with tin catalysts. The catalyst provides selective catalysis and is stable in the polyol component of two-component waterborne polyurethane systems.
Selectivity Studies
Many two-component waterborne urethane formulators are challenged to find a catalyst that is stable in the water-containing polyol component and can selectively catalyze the polyol/isocyanate reaction in the presence of water. Waterborne two-component urethanes are typically formulated to have a large excess of NCO; typically the NCO:OH ratio is about 1.5:1.0. This is to compensate for isocyanate that will inevitably react with water.
Final film properties can be greatly affected by variations in the final poyurea/polyurethane content. In the absence of gassing, polyurea can contribute some desirable film properties, such as hardness and chemical resistance.
However, as more polyurea is generated in an aged catalyzed paint, the paint's workable time is reduced because of excess gassing. Workable time, or pot life, is measured not only by viscosity increase (or foaming in the can), but also by gloss reduction of films cast with aged paint. As more water reacts with isocyanate in the can, the gloss of cast films reduces. This is likely due to incompatibility of the polyurea and polyurethane products causing some phase separation, as well as the increased presence of trapped CO2 in the film.
Several methods can be used to quantify catalyst selectivity in a two-component waterborne urethane system. Catalyst selectivity can be indicated by in-can paint stability, properties of cured films (particularly gloss) and Fourier transform infrared spectroscopy (FTIR) analysis. The formation and reduction of functional groups, such as urea and isocyanate groups, can be monitored with FTIR. An FTIR method was used to compare the selectivity characteristics of K-KAT® XK-6143 and DBTDL in a two-component waterborne urethane system.4 The FTIR results suggest that K-KAT XK-614, a newly developed zinc complex, is able to more discriminately accelerate the polyol/isocyanate reaction in the presence of water. Paint stability and film properties were evaluated in the following study to confirm FTIR results.
Catalysis of a Pigmented Two-Component Waterborne Urethane System
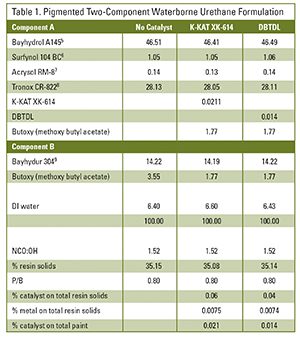
DBTDL and K-KAT XK-614 were compared in a pigmented two-component waterborne urethane system. The levels of DBTDL and K-KAT XK-614 (0.04% and 0.06%, respectively, based on total resin solids) contributed equal metal content. Both catalysts have sufficient hydrolytic stability to allow incorporation on the aqueous polyol side. The catalysts were blended with a co-solvent and added to the polyol component under agitation. Co-solvent was used to decrease the risk of weighing errors and to facilitate incorporation. K-KAT XK-614 is not water soluble and high levels without a co-solvent could destabilize the aqueous dispersion (see Table 1).
Films were cured under ambient and elevated temperature conditions. The baked films were cured at 80˚C for 30 minutes. Dry times of ambient cured films were tested with circular recorders. Hardness development was monitored for a week with a Konig pendulum hardness tester. Final film property testing also included methyl ethyl ketone (MEK) resistance and gloss analysis. Selectivity of DBTDL and K-KAT XK-614 was compared by measuring the gloss of films that were cast with aged paint.
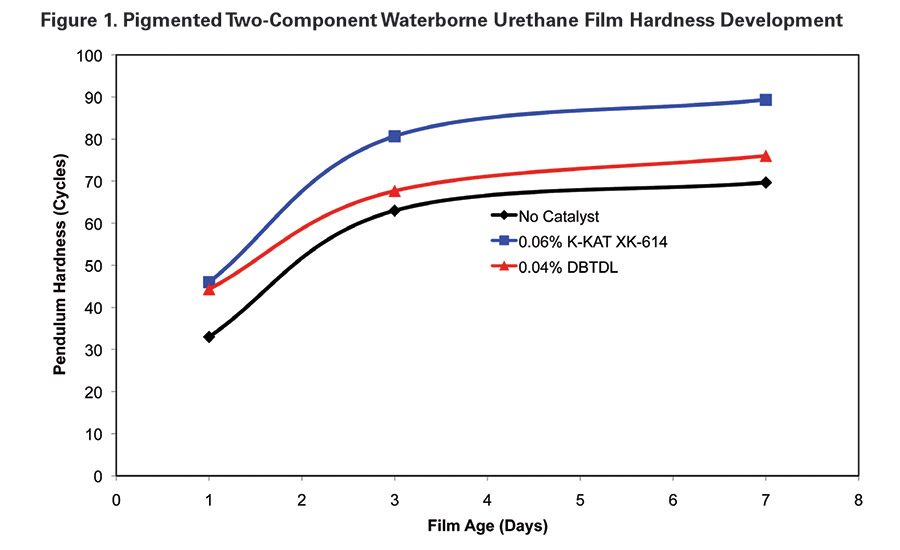
Hardness development of the ambient-cured films demonstrates the activity of K-KAT XK-614. The XK-614 film developed higher initial hardness after one day of ambient cure and continued to maintain a higher hardness through the test period. MEK resistance of films cast with both of the catalyzed systems reached 100+ double rubs. The uncatalyzed films had a heavy mar at 100 double MEK rubs (see Table 2 and Figure 1).
Ambient Cure
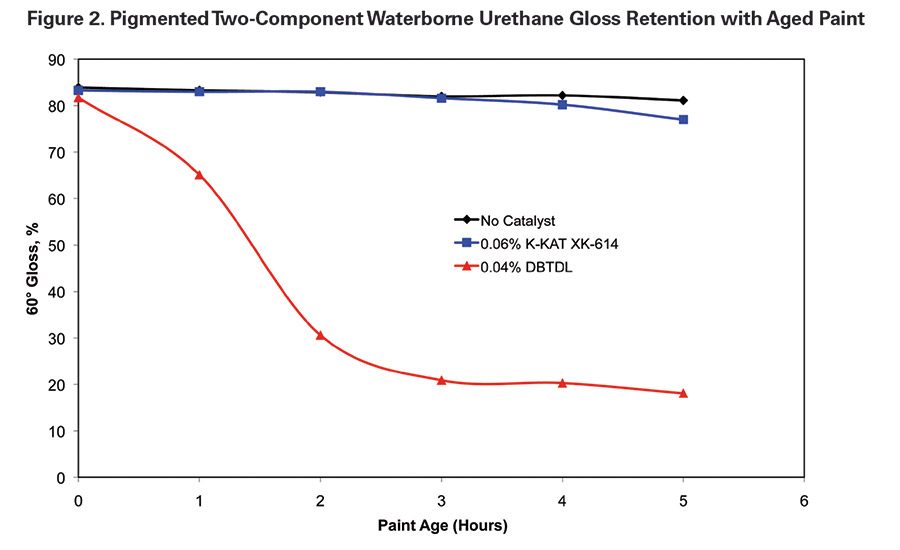
The selectivity of DBTDL and K-KAT XK-614 was compared by measuring the gloss of films that were cast with aged paint. Films were cast immediately after mixing the two components and on one-hour intervals after mixing. The films were baked for 30 minutes at 80˚C to step up the testing process.
The K-KAT XK-614-catalyzed paint was more stable than the DBTDL-catalyzed paint. The gloss of DBTDL-catalyzed films was significantly reduced even after the first hour of aging. The gloss of films made with the K-KAT XK-614 paint started to decrease slightly after the paint was aged for five hours (see Table 3 and Figures 2-3). Figure 3 compares a K-KAT XK-614 film that was cast with paint aged for four hours to a DBTDL film that was cast with paint that was aged two hours.
Hydrolytic Stability of K-KAT XK-614 in a Clear Two-Component Waterborne Urethane System
The formulation used to determine hydrolytic stability of K-KAT XK-614 was a clear version of the pigmented formulation. The XK-614 was stored in the aqueous polyol component at 50˚C for five weeks. The aged polyol containing the catalyst was then blended with the isocyanate and films were cast.
Pendulum hardness was measured after baking at 800˚C for 30 minutes and monitored while air drying for one, three, and seven days. The hydrolytic stability of K-KAT XK-614 was excellent on heat aging in the water-containing polyol component since it remained effective for hardness development, and no detrimental effect on gloss was noted (see Table 4).
K-KAT XK-614 can effectively accelerate the polyol/isocyanate reaction in waterborne formulations, although the catalyst is not water soluble. Therefore, XK-614 must be properly incorporated. If possible, the catalyst should be added to a hydrophobic phase of the polyol component before water is added. When adding to an aqueous polyol component, XK-614 should be pre-blended with a co-solvent and added with agitation. The catalyst is soluble in a variety of ester, ketone, alcohol, glycol, glycol ether, and aromatic and aliphatic solvents.
Non-Coatings Applications
In the area of elastomer, adhesive, and other non-coating urethane applications, catalyst requirements can vary depending on cure conditions and formulation components. Urethane elastomer applications can range from soft microcellular foams to rigid structural composites. Issues related to catalyst selection for these systems include selectivity (e.g., gassing), latency and activity stability.
Achieving the full cure profile of mercury is a challenge. In the field of urethane elastomers and adhesives, organomercury compounds provide excellent "snap cure" and selectivity, but they are also very toxic. Organomercury compounds are known to provide a long induction period followed by a sharp gelation profile of two-component urethane systems.10
Many industrial adhesive, sealant and castable elastomer applications often require the "snap cure" reaction profile that mercury catalysts can provide. Government regulations restricting the use of organomercury compounds compel formulators to search for alternative catalysts that can mimic the performance of mercury catalysts. The following studies demonstrate performance characteristics of catalysts that can be considered alternatives to mercury.
Gel Profile Test Methods
The type of test method used to determine the gel profile mainly depends on the thermodynamics of the reaction. For the following studies, a rheometer and a viscometer were used to evaluate soft elastomer and adhesive systems that did not generate strong exotherms even when mixed in a large mass. A temperature recorder was used to analyze a more exothermic system. Systems that produce hard, non-flexible product are more likely to generate strong exotherm during the curing process, while systems that have more elastomeric properties generate less exotherm.
Gel Profile Studies of Weak Exothermic Reactions
Analyses of systems that did not generate strong exotherms were conducted with rheometers or a viscometer. The rheometers used in these studies were TA Instruments' AR-1000 and AR-2000, which are capable of providing quantitative gel times under essentially static conditions using an oscillation method. Performing gel tests under static conditions eliminates potential variations caused by over-shearing the material.
The oscillation method generates modulus data. G' measures the elastic, or solid, tendency and G" measures the viscous, or liquid, tendency. Gel times are quantitatively measured at the cross-over point of G' and G", beyond which the sample is considered "primarily solid." For clarity, Figures 4, 5 and 6 only include the G' plots, which indicate structure development.
Urethane Elastomer
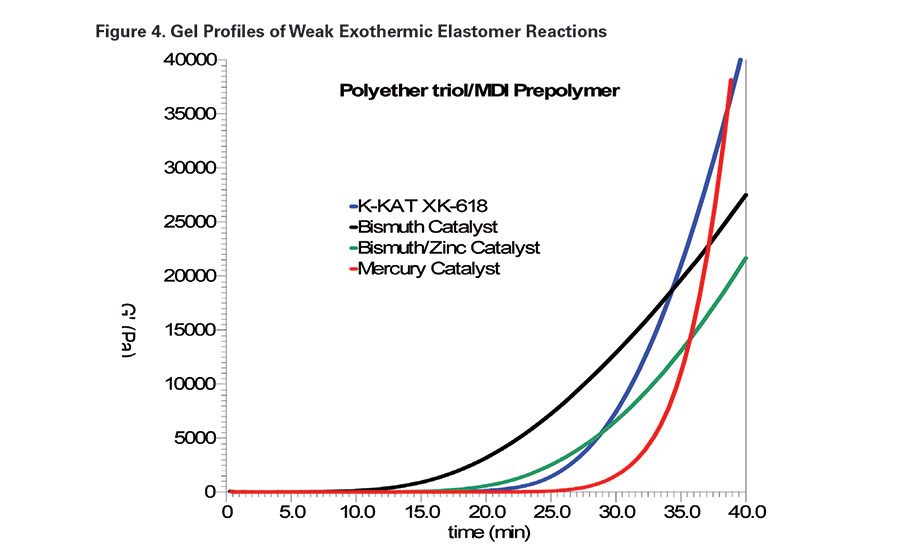
The polyol selected for this study was Poly-G 76-120,11 a polypropylene oxide triol with a hydroxyl equivalent weight of 480. The isocyanate selected was Desmodur E743,12 a polyisocyanate prepolymer based on MDI with an NCO equivalent weight of 525. The newly developed mercury-alternative catalysts, K-KAT XK-604, K-KAT XK-617 and K-KAT XK-618, contain several metal carboxylates. Gel profiles of systems catalyzed with these metal carboxylate "cocktails" are comparable to gel profiles of mercury-catalyzed systems.
An organomercury catalyst13 was used as the primary control in this study. It is 20% diphenyl mercury acetate in a non-reactive diluent. A 35-40 minute gel time target range was established with 0.1% Hg metal, or 0.5% catalyst as supplied. In addition, a bismuth carboxylate catalyst and a bismuth/zinc combination were included as controls. The bismuth catalyst was included to represent a poor gel profile, and the bismuth/zinc catalyst represents a commercial mercury alternative. As previously demonstrated,14 zinc and bismuth can work synergistically to provide improved gel profiles. Figure 4 compares the gel profiles of the system catalyzed with above-mentioned catalysts and with 0.12% K-KAT XK-618 as supplied.
In general, the straight bismuth carboxylate compounds appeared to be more active during the initial phase of the overall polyol/polyisocyanate reaction. Bismuth catalysts also tend to lose activity during the curing process, which often leads to poor through-cure. This deactivation is likely due to hydrolysis of the bismuth catalyst. The bismuth/zinc catalyzed system had a delayed onset point; however, the reaction rate beyond the gel point was still lacking.
In addition to a delayed onset point, the optimum gel profile has a progressively increasing rate of structure development beyond the gel point. As demonstrated with the mercury and K-KAT XK-618-catalyzed systems, such a profile leads to better through-cure.
Urethane Adhesive
As with urethane elastomers, the relationship between cure response and pot life of a two-component urethane adhesive is dependent on the catalyst. The resin system used for the urethane adhesive study was based on a polytetramethylene ether glycol (PTMEG)-MDI prepolymer and a polyether diol.
Catalyzed reaction rates were determined by initially preparing 100-g samples in 250-mL beakers. A 20-mil film was cast onto aluminum panels and baked for five minutes at 110˚C. Cure response, or tack, was determined by touching the film with a glass rod. The films were considered tack-free when the glass rod left no mark.
Viscosity increase was monitored with a Brookfield DV-II+ Pro viscometer using 150-g samples. Viscosity was recorded at one-minute intervals. Figure 5 compares the viscosity profile of the system catalyzed with a bismuth carboxylate and with K-KAT XK-618. Catalyst levels were optimized to obtain a tack-free film and maximum pot life. The system catalyzed with K-KAT XK-618 provided equal cure and longer pot life than the bismuth carboxylate-catalyzed system.
Gel Profile Studies of Strong Exothermic Reactions
Analysis of the temperature increase during the reaction of strong exothermic systems provides valuable profile information. In this case, relatively large samples were used to allow for the thermodynamics to take effect. The amount of formulation used for each catalyst evaluation was 250 g. The materials were mixed before pouring 100 g into two 100-ml plastic beakers.
A thermocouple probe was immediately placed into one of the beakers. The probe was fixed to a stand to maintain a constant depth in each sample (approximately 1.3 cm from the top). The thermocouple was connected to a temperature recorder that was interfaced with a computer. Approximate gel times were based on physically probing the material in the beaker without the temperature probe. This method generated temperature curves that seem to closely correlate with the gel profiles.
The increasing temperature of a strong exothermic reaction can activate, or increase the activation, of catalysts that are fully or partially inhibited. In this case, heat-activated catalysts can potentially enhance the system's snap cure response. To demonstrate heat activation of a catalyst using this process, a study was conducted using a low-molecular-weight polymeric MDI with a polyether triol that was capable of reaching reaction temperatures above 140˚C. The two reaction temperature profiles in Figure 6 compare the system catalyzed with a straight bismuth carboxylate catalyst and K-KAT XK-614. The reaction profiles suggest that the XK-614 becomes increasingly active as the temperature approaches 80˚C, providing more snap cure.
Summary and Conclusion
A series of newly developed catalysts are being used in a variety of CASE applications. These catalysts are capable of providing similar or exceptional performance when compared to tin and mercury catalysts, but without the associated environmental concerns. In particular, K-KAT XK-614 is a versatile catalyst that is effective in waterborne coatings and elastomer systems. It has good hydrolytic stability and more tendency to accelerate the polyol/isocyanate reaction in the presence of water, resulting in less gassing.
In non-coating urethane applications, the mixed metal "cocktail" K-KAT catalysts have demonstrated similar reaction profiles compared to mercury, and exceptional reaction profiles compared to bismuth and bismuth/zinc combinations.
For more information, contact King Industries Inc. at Science Rd., Box 588, Norwalk, CT 06852; phone (203) 866-5551; fax (203) 866-1268; email info@kingindustries.com; or visit www.kingindustries.com.
Acknowledgements
The authors wish to thank Marvin Blair for evaluations of the new catalysts in various coating systems, and King Industries for permission to publish this work.
Editor's note: This article is based on a paper given at the Center for Polyurethanes Industry's (CPI) Polyurethanes 2010 Technical Conference, September 2010.
References
1. Florio, J.J., Miller, D.J., Handbook of Coatings Additives Second Edition, Marcel Dekker/CRC Press, New York, 2004.
2. Florio, J.J., "Non-Tin Metal Catalysts for Urethane Coatings," Paint & Coatings Industry, 1997, 13 (10) 110.
3. K-KAT catalysts are products of King Industries, Inc.
4. Ravichandran, R., Hsieh, B., Florio, J.J., Coughlin, R.D., "New Tin Free Organometallic Catalysts for Urethanes," 2010 ACS, Charlotte, NC.
5. Bayer MaterialScience, Bayhydrol A145: Aqueous hydroxyl-functional polyacrylic dispersion, solids % = 45, OH eq. wt. ~ 1,145 as supplied.
6. Air Products, Surfynol 104 BC: Surfactant, 50% in Butyl Cellosolve.
7. Rohm & Haas, Acrysol RM-8: Rheology modifier.
8. Tronox Incorporated, TiO2 pigment.
9. Bayer Material Science, Bayhydur 304: Hydrophilically modified, aliphatic polyisocyanate based on HDI, solids % = 100, NCO eq. wt. ~ 230.
10. Robins, J., Appl. Polym. Sci., 9 (1965), 821.
11. Arch Chemical, Poly-G 76-120: polypropylene oxide triol, hydroxyl equivalent weight = 480.
12. Bayer MaterialScience, Desmodur E743: polyisocyanate prepolymer based on MDI, NCO equivalent weight = 525.
13. CasChem, Cocure 44 and Cocure 55.
14. Arenivar, J.D., "Viscosity Control of Curing Elastomers Using BiCAT® Catalysts," Polyurethane, 1995, September 26-29, 1995, pp. 131-136.
Originally published on August 1, 2011