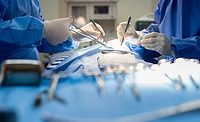
Avery Dennison Medical Solutions, a division of Avery Dennison Corp., has reported that independent testing confirmed that the new Avery Dennison Medical Solutions chlorhexidine gluconate (CHG) adhesive delivery system (ADS) provides antimicrobial efficacy across a range of bacteria and yeast. These data demonstrate that the challenge of incorporating CHG within a solvent acrylic adhesive has been successfully addressed.
The new CHG ADS is transparent and reportedly allows an access or incision site to be seen, a critical parameter for vascular access and post-op dressings. The formulation will ultimately lend itself to use as a transparent film dressing that will make it easier to monitor sites such as catheter insertions.
In cytotoxicity tests, the CHG ADS formulation reportedly outperformed two commercial products containing CHG by exhibiting a grade 0 cytotoxicity profile, compared with a grade 3 profile for the two commercial formulations. These preliminary tests show that the new CHG ADS may be appropriate for applications where the spread of infection is a concern and moisture management and transparency are required, such as the securement of IV catheters, surgical incision films, and post-op dressings.
“Until now, there has been limited success in incorporating CHG into a solvent adhesive formulation,” said Anne Wibaux, Pharm. D, Avery Dennison Medical Solutions principal scientist. “Other products impregnate the CHG molecule into a different matrix, but do not formulate it into an adhesive.”
For more information, visitwww.medical.averydennison.com.
The latest news and information
Content focused on adhesive & sealant manufacturing, formulations and finished products
SUBSCRIBE TODAYCopyright ©2024. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing