Here, the behavior of the base emulsion is separated from that of the formulated product. The difficulty is that the latex must be coated onto a release liner without the aid of a wetting agent. Through some trial and error, it was found that an acceptable film can be coated with the use of certain rheology modifiers that were found to have a negligible affect on the fragmentation behavior of the PSA.
Initially in this study, 25 label-grade and general-use adhesives were investigated. This provided a broad range of monomers, surfactants and properties. Previously published studies had claimed correlations between screening removal efficiencies and performance properties of the adhesive, but these were not evident in this study.15 Guo et al. reported that “highly hydrophobic polymers yielded larger adhesive particles that were easily removed during the screening operation.”16 A relationship was observed between the composition of the adhesive polymer and its removal.11
The commercial PSAs studied represent 25 different monomer combinations using 15 different monomers. It was seen that those PSAs containing both vinyl acetate and acrylic acid monomers provided poor removal efficiencies. In addition, it was found that the PSAs demonstrating only modest removal efficiencies contain either vinyl acetate or acrylic acid. These results indicate that the combination of particular monomers produces a critical change in the property controlling the fragmentation of the adhesive, and this change is not apparent from the mechanical, surface, or performance properties of dry films.
Given the likely tie between mechanical strength and the tendency to fragment, studies were designed to examine the impact of moisture on the strength of PSA films. A convenient method for gauging the strength of a material is through tensile testing. However, given their highly viscoelastic nature, this is a difficult task for PSA films; it is further complicated by the need to be carried out in a temperature-controlled, aqueous environment. For our approach,11 tensile samples are produced by coating the PSA film over two separate pieces of 25-mm-wide PET films that are placed against each other to form a continuous substrate for the adhesive. All of the films cast for this study are targeted for 1 mil (25.4 µm). The ends of the PET are not coated and serve as gripping tabs. The tests are carried out in a temperature-controlled water bath at a crosshead speed of 10 mm/min.
In using this test to analyze the commercial PSA films, the same trends identified in the removal efficiency data were observed. Kinetic studies (tensile strength vs. soaking time) showed that the changes in strength induced in PSA films occurred in the first few seconds of being submerged into the water.11 These changes were substantial, but the strength quickly stabilized. It was also found that soaking PSA films prior to repulping did not change their removal efficiencies. These results indicate that once water-based acrylic films are placed in water, their properties can be very different from those of the dry films.
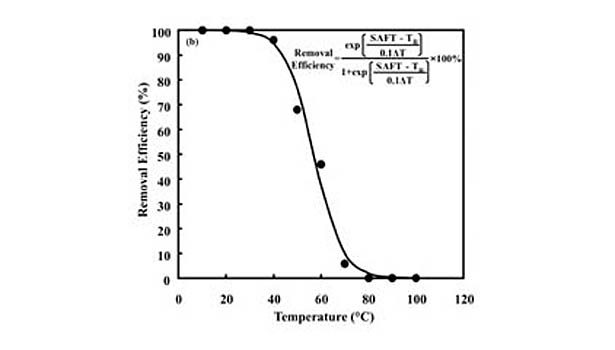
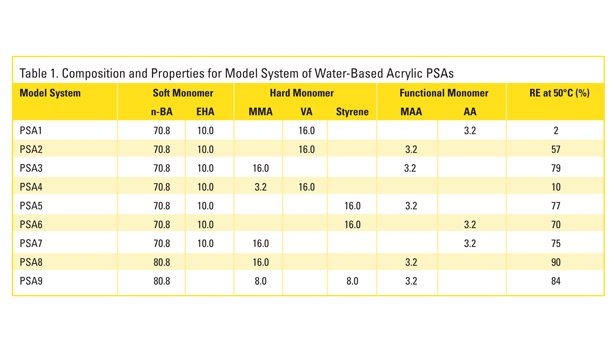
The information obtained from studying the commercial PSAs led to the development of model acrylic water-based adhesive emulsions, which were synthesized using the same additives and approach. The model emulsions are representative of those used in PS labels. They are composed mostly (~ 81 mass%) of soft monomers n-butyl acrylate (BA) and 2-ethylhexyl acrylate (EHA); various combinations of the hard monomers (~ 16-19 mass%) methyl methacrylate (MMA), vinyl acetate (VA) and styrene (STY); and the functional monomers (~ 0-3 mass%) acrylic acid (AA) and methacrylic acid (MAA). The specific monomer composition and various properties for the model PSAs, including removal efficiencies (RE) measured at 50°C, are shown in Table 1.
As was the case for the commercial formulations, a comparison of removal efficiencies with the monomer compositions indicates that the combination of VA and AA results in films with the lowest removal efficiencies. Keeping everything else in the formulation fixed, replacing VA with MMA or STY increases removal efficiency. Also, replacing AA with MAA increases removal efficiency substantially. The experimental Log Kow for the monomers VA, MMA, STY, AA and MAA, where Kow is the octanol-water distribution coefficient, are 0.73, 1.38, 2.95, 0.35 and 0.98.17 Log Kow has been shown to correlate with measures of a chemical’s aversion to water; thus, it appears that the results are generally consistent with the hypothesis that increasing hydrophobicity enhances the removal efficiency of the adhesive polymer.18
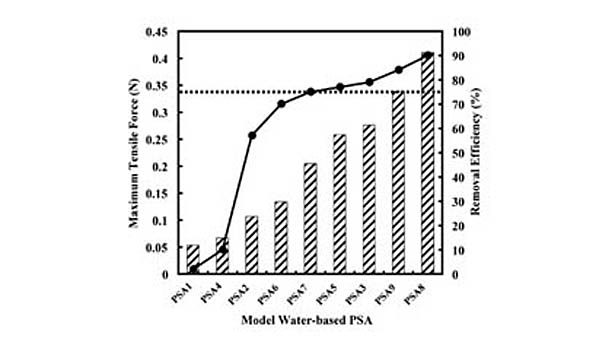
However, it should be emphasized that only the presence of the most hydrophilic of these monomers results in low removals, and both VA and AA are required to produce the extremely poor efficiencies. Avoiding these and similar monomers appears to be of key importance in making water-based PSAs more recycling compatible. Furthermore, increasing removal efficiencies does not appear to be simply a matter of using the most hydrophobic monomers. For example, removal efficiencies were found to be reduced by replacing the harder BA (Tg=-54°C, Log Kow=2.36) with the significantly more hydrophobic EHA (Tg=-80°C, Log Kow=4.09). It appears that under certain circumstances (e.g., monomers possessing sufficiently high hydrophobicities), the hardness of the monomer is of greater importance, and in general, the wet strength of the PSA film is what ultimately determines its fragmentation behavior and thus removal efficiency. This is demonstrated in Figure 3, which shows the wet-tensile strength of the model PSAs, along with their screening removal efficiencies.
Recycling Pressure-Sensitive Products