Producers and converters of goods are constantly looking for process improvements that allow them to increase productivity. Ultraviolet/electron beam (UV/EB) curing can increase the speed of production while decreasing the overall environmental impact. While UV/EB curing is well-known to producers and converters of essentially two-dimensional surfaces—such as flooring, optical media, and printed surfaces—the technology can enable process improvements in less obvious applications, like sealants and potting compounds. Industrial sealants and potting compounds, such as those used in equipment, automotive, and electric grid construction, have thus far been considered niche applications.
These applications have a different set of requirements than other UV/EB areas. In traditional UV/EB applications, the coating, adhesive, or ink is typically applied at a very thin coat weight and provides surface modification or protection. In the case of sealants, the applied chemistry is expected to bridge the gap between solid surfaces and possibly tie them together. The sealant should have a certain surface hardness that will allow compression during assembly, and it should be able to withstand mechanical stresses. Potting compounds fill in pre-determined areas to protect the contents or users of the goods.
Sealants and potting compounds are expected to perform in more adverse environments than standard UV-cured systems. These systems could be exposed to high temperature and high humidity for extended periods of time. There is also potential exposure from liquids, such as water, cooling fluids or lubricating oils. It is therefore necessary to understand the resistance of the UV-cured system to all of these potential exposures. As with any system, changes in mechanical or adhesion properties over the service life are undesirable. Understanding how certain monomers and oligomers can hold up under these adverse conditions becomes critical in assembling the best solution possible.
LEARNING WHAT WORKS
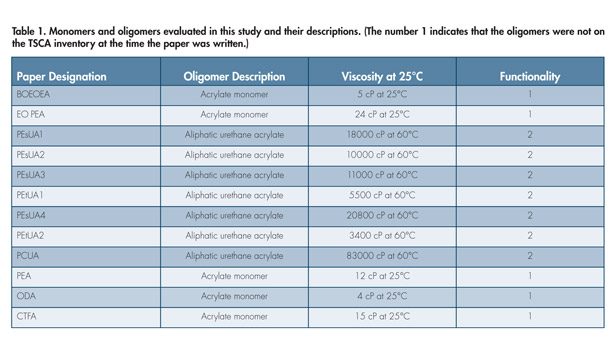
A study was undertaken to understand the key differences in monomer and oligomer backbone structure as heat aging is applied to the UV-cured sealant. The focus of the oligomer evaluations was on aliphatic urethane acrylates, as these backbones allow for differentiation of the molecular weight, backbone chemistry and functionality. By varying these oligomer design elements, applications chemists can achieve a wide range of tensile, viscoelastic, solvent resistance and liquid properties. A plethora of monomer structures could potentially be used in these applications. Table 1 shows a list of the prospective products.
All of the monomers selected for the final parts of this study were monoacrylates. Earlier research revealed that any higher functional monomer, such as diacrylates and triacrylates, introduced too much hardness to the systems. The higher functional acrylates could be useful at additive levels to tweak in properties, but they will not be the main diluents.
Different monomer and oligomer chemistries were examined by adopting a standardized formulation and changing compositions. The main evaluation techniques for this study were Shore A hardness and tensile testing. These two methods allow for an evaluation of the surface and bulk stiffness of the UV-cured systems. Taken individually, they tell only a portion of the story of what is happening to the properties. Taken together, they paint a clear picture of how the system is changing over exposure time.
EXPERIMENTAL DETAILS
All of the samples were cured with a Fusion UV Systems 600 W/in D lamp at 25 ft per minute for a total UV irradiation of 3.2 J/cm2. Samples for tensile testing were prepared by drawing down the samples on Q-Panel A-612 6 x 12-in. mill-finished aluminum panels. Tensile testing was performed according to ASTM D 882 on an Instron 5543 tensile tester equipped with Bluehill analysis software. Samples for Shore A hardness were prepared by creating a 7-mm-thick well on an aluminum panel using Frost King vinyl foam weatherseal. Shore A hardness was performed using a tester from The Shore Instrument & Manufacturing Co., Inc. and ascribing to ASTM D 2240. All of the samples were exposed at 85°C, with either 25% humidity (room) or 85% humidity. The 85°C/85 RH exposures were performed in a Hotpack constant temperature/constant humidity chamber, model 434304. The 85°C/25 RH exposures were performed using a LabLine Imperial V oven.
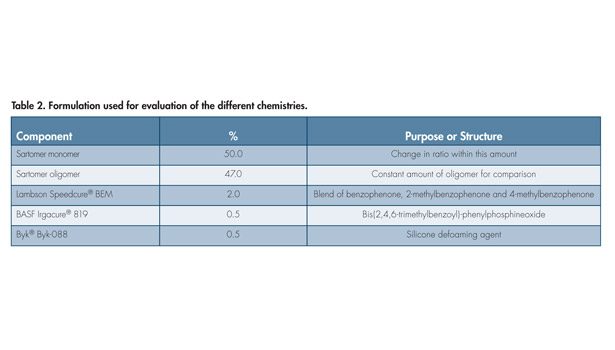
To understand what chemistries can withstand the exposure testing with only minor changes, the study began by looking at different monomers that have shown some utility. All of the evaluations were based on the formulation from Table 2, with PEtUA1 used as the oligomer. The type and ratio of the monomers used at 50% in the formulation was changed. The two monomers used in the formulation were BOEAEA and CTFA. The formulations were all applied and UV-cured at room temperature using the prescribed lamp and energy. The samples were then tested for hardness and tensile properties. Separate samples were put in the Hotpack and LabLine ovens to expose them to the different conditions for 168 hours.
ANALYZING RESULTS
The results reveal several insights. First, as the amount of CTFA is increased in the formulation, the Shore A hardness of the formulation increases. This is mainly due to the glass-transition temperature (Tg) of CTFA vs. that of BOEAEA. When UV-cured, BOEAEA yields a photopolymer with a very low Tg of -73°C (by DSC), whereas CTFA yields a Tg of 32°C (by DSC). Simply changing the Tg of the monomer affects the overall Tg of the cured system. In this case, increasing the Tg moves the cured sealant formulation into a more glassy state with higher modulus. As the formulation that contains only CTFA as the monomer shows, the modulus and Shore A increased dramatically.
Second, increasing the amount of CTFA in the formulation actually decreases the heat stability of the formulation. When CTFA was used at up to 30% in the formulation, the Shore A hardness and modulus of the dry and moist exposed polymers did not vary by more than 10% from the unexposed sample. Formulations with greater than 40% of CTFA increased dramatically in hardness and modulus when exposed to either dry or moist heat. The percentage of CTFA in a formulation would therefore be limited to avoid stiffening of the UV sealant during exposure. To further confirm that CTFA causes stability issues, PEA was substituted for the monomer and evaluated.
Comparing Figure 3 to Figure 1 and Figure 4 to Figure 2, one can see how PEA performs vs. CTFA. Substituting PEA for BOEAEA in the formulation gave an even, stepwise increase in the Shore A hardness of the UV-cured formulations. After dry and moist heat exposure, the hardness remained within 10% of the unexposed sample. Interestingly, increasing the amount of PEA in the formulation did not dramatically increase the modulus of the system. After aging, the modulus of the samples stayed within 10% of the unexposed samples. Quite simply, the evidence suggests that formulations based on PEA offer superior dry and moist heat aging vs. those based on CTFA.
As those who have experience with UV- or EB-cured formulations know, the oligomer is the major contributor to the overall properties of the system. The monomer utilization and properties described previously can be used to modify the base properties of the oligomers themselves. In this work, urethane diacrylate oligomers showed the best utility for sealant and potting compound applications. Use of these oligomers allows for changes in backbone structure and molecular weight, as well as good analysis of key properties. The initial evaluation was to look at the properties of a polyether, polyester or polycarbonate backbone aliphatic urethane diacrylate of similar molecular weights. In this, the contribution of the specific backbones to the overall properties of the system was isolated.
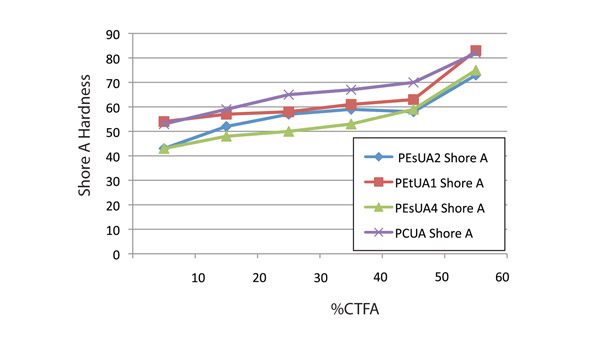
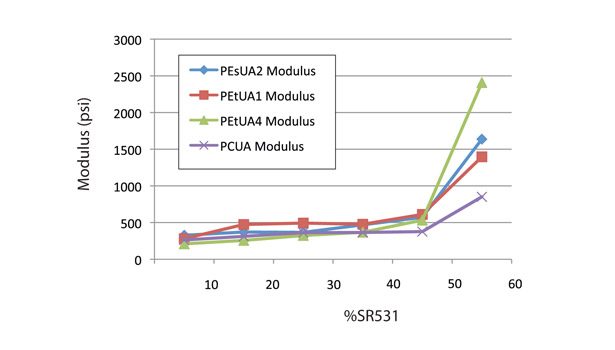
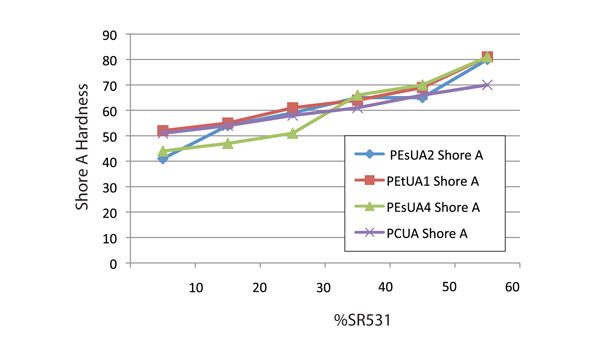
Figure 6 shows that at CTFA levels of less than 40%, all the formulations showed similar modulus. By contrast, Figure 5 shows some differentiation in the oligomer backbones. The polycarbonate-based oligomer has a significantly higher Shore A hardness than the polyester- or polyether-based ones. The polyether-based oligomer, PEtUA1, actually showed higher Shore A and modulus than the standard polyester- and hydrophobic polyester- backboned PEsUA1 and PEsUA4.
After exposure to the 85°C/85 RH conditions for 168 hours, all of the sealants showed changes in their Shore A hardness. PEtUA1 showed the best stability, as the Shore A for the formulations with greater than 40% CTFA maintained their hardness within 10% of the unexposed sealant. The good stability is probably due to its balance of hydrophobicity and hydrophilicity, which allows water vapor to pass through the UV-cured film with minimal effect. Also, the lack of ester linkages within the oligomer backbone eliminates one possible area of failure under these conditions.
The polycarbonate backbone showed a slight decrease in hardness. The standard and hydrophobic polyesters both showed a dramatic increase in hardness after the exposure. For these two products, note that the formulation does not contain organic or inorganic acids. Had the formulation contained those compounds, the polyesters might have hydrolyzed under the high heat and humidity conditions. Therefore, when the cured sealants were exposed to high temperature and humidity, the polyether backbone maintained its properties better than the other backbone chemistries.
As with any study of urethane acrylate oligomers, it is interesting to examine the effect of molecular weight on the properties of the system. The core of the urethane acrylate oligomer itself is changing in molecular weight and is effectively increasing the length between crosslinks. Of the PEsUA1 and PEsUA2 products, the PEsUA2 has a significantly higher molecular weight, yet only shows a large decrease in Shore A hardness once high levels of PEA are used in the formulation. At lower levels, the molecular weight differences have little effect. The PESUA3 is dramatically higher in molecular weight, as demonstrated by how much softer the sealant is when based on that oligomer. In addition, the PEsUA3-based formulation has twice the viscosity of the other two. In essence, increasing the molecular weight of the oligomer is an effective tool to manipulate the overall hardness of the system.
ADDITIONAL TESTING
It is one thing to be able to withstand temperature and humidity conditions for a period of time, but many of the targeted applications could expose the photopolymer to liquid chemicals. Therefore, it is important to understand how well the UV-cured system can withstand long-term chemical exposure. To that end, we changed the formulation based upon what we learned from earlier work.
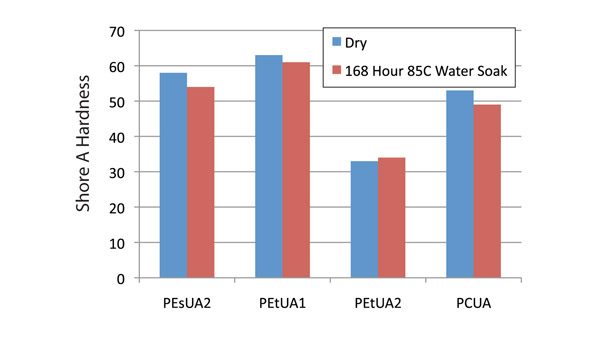
Of all of the testing so far in the project, this test yielded the most surprising results. Acrylate coatings soaked in hot water for extended periods of time will typically exhibit some changes in their physical properties. In the case of these samples, the high film thickness would be expected to see a much more pronounced effect.
The samples were tested immediately after removal from the water so any water trapped in the photopolymer could be seen through changes in its properties. In fact, after 168 hours (one week) in 85°C water, the effect on the hardness of the photopolymer was negligible. All of the Shore A hardness levels were within 10% of their original values. The cured formulations have a good amount of polarity built into them and would be expected to draw in a significant amount of water during the test. That clearly did not happen and lends to the good utility of UV- or EB-cured chemistries in applications where chemical resistance is needed.
CONCLUSION
This study shows that UV- or EB-cured acrylate chemistries have the necessary performance characteristics for sealant and potting compound applications. Requirements such as hardness, elongation, resistance to dry and humid heat, and chemical resistance can be met when the correct monomers and oligomers are used. Cured photopolymer properties can be predictably manipulated by changing the backbone structures and molecular weight of the various components. Polyether-backboned monomers and oligomers showed the best overall performance and maintained that performance when exposed to both dry and humid conditions.
For more information, call (610) 363-4100, email contact@sartomer.com or visit www.sartomer.com.
Editor’s note: The author presented a version of this article at RadTech 2012.