First produced commercially in 1951, glutaraldehyde provides broad efficacy, is readily biodegradable and formaldehyde free, and is also non-carcinogenic, non-
| Jump to: Genuine Glutaraldehyde |
persistent and non-bio-accumulative, which distinguishes it from many other biocides. This versatile microbiocide offers a host of advantages in controlling microbes found in commercial and industrial settings, and its applications are of use to global formulators, manufacturers and end users in the adhesives and sealants industries.
GLUTARALDEHYDE APPLICATIONS AND FEATURES
Commercial and industrial uses of the glutaraldehyde biocide are varied, including controlling nuisance and hazardous microbes found in oilfield systems, metalworking fluids, pulp and paper facilities, animal biosecurity, and medical equipment hygiene. In addition, glutaraldehyde can be applied as a preservative to various water-based consumer/industrial products, such as adhesives and sealants, to protect them from common spoilage bacteria and fungi, thereby extending the shelf life of the manufactured product. The versatility of the glutaraldehyde molecule is further demonstrated in that this particular biocide chemistry can be applied as a rapid-kill microbiocide to process equipment, water sources and raw materials used in manufacturing facilities to minimize microbial contamination and dissemination into the formulated, finished product.
In the U.S., the use rates for glutaraldehyde, like all antimicrobials, are defined on the U.S. Environmental Protection Agency (EPA)-approved label. The legal upper and lower limits for antimicrobial doses are data-based, meaning that efficacy against microbes must be demonstrated throughout the allowed range.
A variety of glutaraldehyde-based products are available on the market. The Dow Chemical Co. and BASF Corp. are the major producers of glutaraldehyde, most commonly as a 50% solution in water, and also hold U.S. EPA registrations for this chemistry. Service companies and distributors may hold sub-registrations (i.e., to sell Dow or BASF products under an alternative trade name) or have registered formulations based on the parent glutaraldehyde products.
Mode of Action
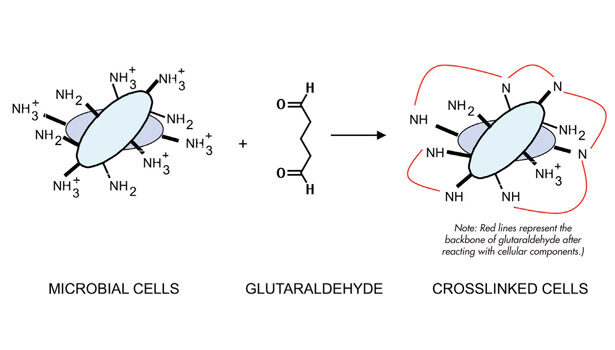
Glutaraldehyde is effective against a broad range of microbes, including Gram-positive and Gram-negative bacteria, Legionella, sulfate-reducing bacteria, yeasts and molds, viruses, and spores.1 Glutaraldehyde is a 5-carbon molecule with two aldehyde functional groups (represented as H-C=O), which are highly reactive toward amines. Amines (represented as –NH2 or –NH3+) are commonly found on the surfaces of microbial cells and proteins. When glutaraldehyde comes into contact with these biological entities, it chemically modifies or crosslinks them (see Figure 1). This inactivates proteins and immobilizes cells, resulting in the death of the microbe.
Environmental Profile
The major pathway for glutaraldehyde breakdown in natural systems is through biodegradation. This chemical is considered “readily biodegradable” by internationally accepted standard test methods (OECD 301).
Occupational Safety
When considering risks to both people and the environment, it is important to differentiate between use concentrations and products in the drum. Drums, totes
When considering risks to both people and the environment, it is important to differentiate between use concentrations and products in the drum. |
and pails of glutaraldehyde should always be handled in a well-ventilated area, preferably at room temperature or lower. It is good practice to use a pump to transfer glutaraldehyde into water systems when possible to help prevent worker exposure. As with all chemicals, proper personal protective equipment (PPE) should always be used.
Guidance on environmental and procedural controls for the safe handling and storage of glutaraldehyde is available.2 At use concentrations, the risks associated with glutaraldehyde, such as skin, eye and respiratory irritation, are significantly mitigated. It is important to note that based on the human health assessments compiled by the EPA, glutaraldehyde is classified as “Not Likely to be Carcinogenic to Humans” by any route of exposure.
A Long, Data-Rich History
Glutaraldehyde was first produced in 1951, and has been registered as an antimicrobial in the U.S. since 1963. In the U.S., glutaraldehyde is regulated under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), as are all other biocides. In recent years, changes to this EPA-administered legislation have required that regulated chemistries be reviewed with a focus on environmental and health risks. In 2007, the EPA issued a Re-registration Eligibility Decision (RED) approving glutaraldehyde for continued use. The RED document, which is publicly available, contains a great deal of data on use patterns, hazards and risk assessments.3
Glutaraldehyde is supported in Europe under the framework of the Biocidal Products Directive 98/8/EC (BPD). This directive concerns the authorization and placing on the market for use of biocidal products within the Member States, the mutual recognition of authorizations within the community and the establishment at the community level of a positive list of active substances that may be used in biocidal products.
BPD dossiers for glutaraldehyde are currently under review for a variety of uses, including public health hygiene, veterinary hygiene, in-can preservation, cooling water and slimicides. In addition, many glutaraldehyde-containing formulations are approved for use in oilfield applications in the North Sea and, in several cases, glutaraldehyde has received the highest available rating.4
GENUINE GLUTARALDEHYDE
As stated previously, the major manufacturers of glutaraldehyde are Dow and BASF. Both companies have many years of experience and expertise in producing high-quality, reliable products. However, it has become increasingly common in some markets to find low-quality products that are advertised and sold as glutaraldehyde.
This trend is troubling for a number of reasons. The most common substitute for glutaraldehyde in these counterfeit formulations is formaldehyde (FA). This substitution alters the environmental and handling profiles of the product. It also means that the producers of this counterfeit product are charging buyers for glutaraldehyde, but selling a much less expensive product. Without sophisticated analytical chemistry capabilities, it can be difficult to distinguish between genuine glutaraldehyde and a product mixed with FA or other aldehydes.
Counterfeit Products Have Altered Safety Profiles
The 2011 edition of the Report on Carcinogens, published by the U.S. Department of Health and Human Services, states that free formaldehyde is “known to be a human carcinogen.”5 Free formaldehyde also has lower exposure limits as defined by OSHA and NIOSH. Therefore, the addition of free formaldehyde to formulations can result in more stringent handling guidelines. Not knowing that a formulation contains free FA could result in improper handling and can be detrimental to worker safety.
Counterfeit Products Pass Titration
The quality of glutaraldehyde is most easily evaluated by a simple titration procedure. This test quantifies the number of aldehyde groups present, and assumes that glutaraldehyde is the only aldehyde-containing compound in the sample. Unfortunately, since formaldehyde and compounds such as glyoxal also contain aldehyde groups, they are titrated in this test as well. A product containing 50% glutaraldehyde is indistinguishable by titration from a product containing 36% glutaraldehyde and 10% formaldehyde. Fortunately, more rigorous types of analyses can distinguish between real and adultered glutaraldehyde.
Identifying Genuine Glutaraldehyde
From a chemical and analytical standpoint, genuine glutaraldehyde and non-glutaraldehyde additives can be identified in the laboratory using high-pressure liquid chromatography (HPLC). This method relies on differences in the overall structures of chemical compounds to separate them from each other (see Figure 2).6 From a practical and commercial standpoint, the best way to ensure that the product in your warehouse is genuine glutaraldehyde is to purchase it from Dow, BASF, a trusted distributor of Dow- or BASF-produced glutaraldehyde, or from a reliable service company.
SUMMARY
Glutaraldehyde is a highly effective, broad-spectrum antimicrobial for various commercial and industrial treatment applications. It has a long history of successful industrial use and is backed by robust efficacy, and environmental and safety data. However, in today’s marketplace, users of glutaraldehyde must be vigilant to make sure they are getting genuine glutaraldehyde and not a counterfeit product.
For more information, contact the author at hrmcginley@dow.com or visit www.glutaraldehyde.com.
To learn more, listen to our podcast with Heather McGinley.
AUTHOR’S NOTE
The author would like to thank Jon Raymond, Ph.D., senior microbiologist and customer application specialist at Dow Microbial Control, for his contributions to this article.
REFERENCES
1.
Paulus, Wilfried, Directory of Microbicides for the Protection of Materials-A Handbook, Springer-Verlag, 2005, www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_bookid=1707&VerticalID=0.
2.
(a) The Dow Chemical Co., “Glutaraldehyde Safe Handling and Storage Guide,” Form No. 253-01338-06/01/03, Midland, MI, 2003; (b) The Dow Chemical Co., “Spills, Deactivation, and Disposal of Glutaraldehyde,” Form No. 253-01443 01/25/08 PS, Midland, MI, 2008.
3.
See www.epa.gov/pesticides/reregistration/status_page_g.htm for RED documents for EPA-registered pesticides, including antimicrobials.
4.
See, for example, the UK ratings as published by Cefas, www.cefas.defra.gov.uk/industry-information/offshore-chemical-notification-scheme.aspx.
5.
Report on Carcinogens, Twelfth Edition, National Toxicology Program, Department of Health and Human Services, 2011, http://ntp.niehs.nih.gov/go/roc12.
6.
Appiah-Amponsah, E.; Jevey, H.; DeBruhl, S.; Sharley, Dorota, “Chromatographic-Based Approaches for the Identification of Adulterants Present in Dow Genuine Glutaraldehyde Products,” paper presented at the 126th AOAC International Meeting and Exposition, Las Vegas, NV, October 1-4, 2012.