Today’s elastomeric joint sealants are quite remarkable. For a relatively low cost, they provide much of the protection against air or water intrusion for many types of assemblies. In these applications, a sealant must adhere tenaciously to the substrates and maintain prolonged flexibility while being bombarded by wind, rain, heat, ultraviolet (UV) light, ice, dirt, and so on. In many cases, the sealants are also expected to maintain their color.
Discoloration of paints, finished plastic components or sheets is a well-documented subject; however, it seems the same cannot be said of sealants. Granted, in terms of surface area in use, sealants account for a relatively small amount. Because of their location and visibility on many assemblies, however, any discoloration is readily apparent and often unsightly.
For the most part, the only solution to this problem is a costly rework of seams to replace any sealant. This may be done in vain, however, if the basic cause of the problem is not identified or understood and the discoloration returns at a later time. It is useful to understand the basic mechanisms by which sealants may eventually discolor. This information may enable formulators to improve their offerings and aid end users in choosing the appropriate sealants for their applications.
Paths to Discoloration
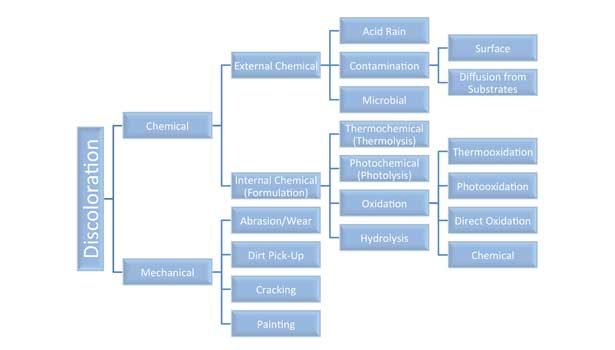
Causes of discoloration can be grouped into two categories: mechanical action or chemical reactions (see Figure 1). Mechanical causes are rather obvious; most often the discoloration involves surface gloss changes, not color. Of the chemical reaction causes, photooxidation and thermooxidation are the most prevalent. In the simplest sense, both routes are similar in that they involve the generation of free radicals on sealant components that subsequently rearrange or react with oxygen or other components to form color centers, also called chromophores. The difference between the two is in the initiation mechanism. Photooxidation is initiated by the absorption of photons of light by certain chemical groups. Thermooxidation is initiated by heat, but this heat can also come from UV light absorption.
Discoloration sometimes appears to be more severe in clear or translucent sealants, since the color is not limited to the surface (or just beneath the surface), as with most pigmented materials. However, discoloration at the surface can be far more severe, not only in terms of aesthetics but also as related to surface integrity. In the case of the latter, if the mechanism leading or lending to discoloration involves degradation reactions of the sealant polymer backbone or other components, breakdown of molecular weight or chain fragmentation can occur and result in eventual chalking. Not only would this chalking result in a dull or matte finish that would further exacerbate any discoloration, but elasticity might also be lost over time. This could result in crack formation.
Chromophores
The pronounced chemical discoloration of sealants, as with many other organic materials, is primarily due to reactions of heat (thermooxidation), light (photooxidation), oxygen, or other chemicals with original formulation components or those introduced as contaminates. These reactions result in the formation of chemical groups in the components known as chromophores. Alternatively or concurrently, the reactions can also cause changes to chromophores already present in the formulation. For the most part, these existing chromophores in sealants are from added pigments, but some formulation components may also have some color. Understanding chromophores and how they are formed is critical to determining how and why sealants discolor.
Chromophores are the parts of molecular entities that selectively absorb light and thereby impart color.1 When chromophores absorb light within the visible range (380-700 nm), they show as the complementary color to that absorbed. For example, if a chromophore absorbs blue light, it will impart a yellow color. For both basic types of chromophores—metal coordination complexes and organic molecules containing p (pi) electron functions—light of certain wavelengths is absorbed by exciting electrons from their ground state into an excited state.
Metal coordination complex (MCC) chromophores, many of which are inorganic pigments, have a general structure with a central transition metal to which ligands, either inorganic or organic, are bound. Most molecules or ions, such as iodine (Iodo-, I-) or hydroxide (hydroxo-, -OH), can serve as ligands. The ligands essentially split the energy levels of the metal atom’s bonding d-orbitals so that, when light is absorbed, an electron can transition from a low to higher energy state at wavelengths in the UV through visible light range (280-780 nm). This mechanism is referred to as a d-d transient.
In some pigments, the chromophores are known as charge transfer complexes. Most are inorganic, but differ from the d-d in that the excitation transition is from the metal to the ligand orbital. The color of chromates with mixed-valence transition metal compounds is due to charge-transfer transitions. The color of semiconductor materials such as CdS yellow is due to the comparatively low energy needed to promote electrons to a delocalized energy band. Generally speaking, all inorganic pigments are either chromates, oxides, phosphates, silicates or sulfides of metals.
Organic chromophores are functional groups that can undergo p p* or n p* in the UV visible light spectrum. Examples of these groups include -C=C- bonds,- C;C- bonds or heteroatoms having non-bonding valence-shell electron pairs, such as nitrogen.
Some simple organic compounds have only one or a few multiple bonds, and while they absorb short-wave UV light, they do not absorb visible light. Because of this, they appear as white or colorless. More complex molecules with conjugated multiple bonds, or “groupings” of many into alternating multiple and single bonds, absorb longer wavelengths. When absorptions occur in the visible light range, the materials are colored. The entire conjugated system giving rise to the color is that molecule’s visible light chromophore.
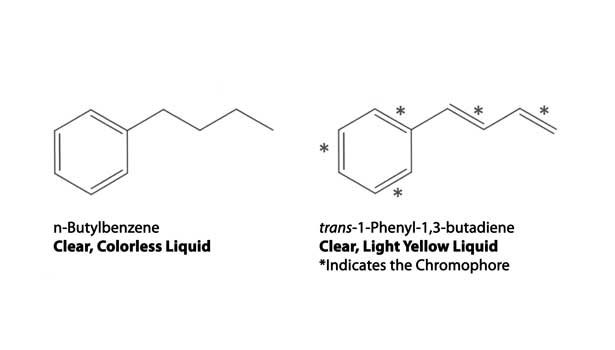
In Figure 2, the effect of conjugation is demonstrated by the comparison of n-butylbenzene and t-phenylbutadiene, the latter having a slight yellow color due to the conjugated double bonds. Figure 2 shows an example of a dependent chromophore, named so because it relies on more than one group to impart color. This is in contrast to independent chromophores (see Figure 3). These groups by themselves are colored or present color in visible light. Examples include, R-N=O, -N=N-, and p-quinonoid groups.2
Auxchromes
Auxchromes are functional groups with a salt-forming unshared pair of electrons that, when attached to a chromophore, shifts its wavelength and intensity of absorption. When these auxchromes are part of a conjugated system of a chromophore, the absorption becomes more intense. Two types of color shifts are possible:
1. A bathochromic or red shift is a shift of an absorption to a longer wavelength (lower energy). Examples of bathochromic groups include electron donors like –OH, -CH3, or -NH2.
2. A hypsochromic or blue shift moves the chromophore to a shorter wavelength (higher energy). Examples of hypsochromic groups include electron acceptors like O=C-H (acetyl).
Measuring Color
The CEI L*a*b* or CEILAB color scale has been an accepted standard for color communication and comparisons since 1976.3,4 In this scale, a color is expressed by three Cartesian coordinates in a color space: L* defines the intensity, a* denotes the red/green color value, and b* denotes the yellow/blue value.
In the context of discoloration, delta (D) or difference values within the scale indicate the change between the original color of a material and its final color after aging or other type of stress. These delta values are expressed as DL* (LAfter Aging – LBefore Aging), Da* and Db*. In many instances, the total color change (or DE) is also calculated and reported. While these delta values are most often used to set manufacturing color tolerances, they can also be used to set allowable degrees of discoloration after simulated or real life aging. Interpretation of delta values is straightforward:
• DL* = difference in lightness/darkness value; (+) means lighter after aging, (-) means darker after aging
• Da* = difference on red/green axis; (+) means redder after aging, (-) means greener after aging
• Db* = difference on yellow/blue axis; (+) means yellower after aging, (-) means bluer after aging
• DE* = total color difference value
Color measurements are made with an instrument called a colorimeter. This optical device essentially responds to color like the human eye, filtering reflected light into its dominant regions of red, green and blue, and comparing intensities before and after reflection. Other methods for color measurements or comparison include the Gardner Color Scale and Yellowness Index. The Gardner Color Scale per ASTM D 1544 is a single-number scale for grading clear liquids samples with color ranging from light yellow to brownish red. Although best-suited for oils, paints and the like, it is sometimes used for clear, low-viscosity sealants. There are 18 visual standards ranging from light to dark, increasing in yellow saturation dominance, and shifting from greenish tint to red tint.5
Yellowness Index (YI), per ASTM E 313, is a single number calculated from spectrophotometric data to describe the changes in color of a test sample from ideal white to yellow. The higher the YI, the more yellow the material.
Chemical Discoloration and Sealant Formulation
As previously stated, the chemical discoloration of sealants is due primarily to the formation of chromophores by reactions of heat, light, oxygen or other chemicals with original formulation components or those introduced as contaminates. Accordingly, knowledge of the makeup of each sealant is needed to understand, predict, and hopefully prevent discoloration.
Single-component sealants are comprised of numerous and diverse components, the specifics of which depend on the basic type. For this discussion, the focus will be on the six most-used, one-component, ambient-temperature applied types:
• Acrylics (water and solvent based)
• Silicones
• Polysulfides
• Polyurethanes (PURs)
• Silane-terminated polymers (STPs); this includes MS polymers, SPURs, MSP, SMP, etc.
• Solvent-borne, rubber-based; typically styrene/rubber block co- or terpolymers
Each formulation is, to a large extent, unique in terms of exact components such as base polymers or crosslinkers. It is possible, however, to describe all with a generic formula.
Polymer Backbone
In this discussion, the backbone of a sealant is the long chain polymer that forms the basic structure of the cured material. It is also the component after which the sealant is usually named; for example, polysulfide sealants are based on linear polysulfide polymers, and PURs are built up from urethane prepolymers. This component typically accounts for 25-80% of a formulation, depending on the chemistry.
Fillers
Fillers are typically inorganic compounds added to reduce cost, improve properties or both. They account for about 10-60% of a formulation. Examples include silicas, calcium carbonate (calcite), talc and clay. Pigment fillers include titanium dixiode, zinc oxide, iron oxides and carbon black.
Plasticizers
At about 10-30% of a formulation, plasticizers are additives that increase the plasticity of a material. Many types of plasticizers are used, but the selection often depends on the particular sealant chemistry. For example, phthalate esters are typically used in 1C PURs, STPs, and some acrylics, while low-molecular-weight poly (dimethylsiloxane) is used in 1C moisture-cure silicones. Additional plasticizers include paraffin oils and chlorinated paraffin oils. Plasticizers are seldom used in solvent-borne rubber-based sealants.
Crosslinkers or Endblockers
The cure mechanism of sealants is specific to the backbone chemistry. Silicones rely on small multifunctional alkoxy-, acetoxy- or oxime- silanes to join the polymer chains together through a condensation mechanism. The typical concentration is 3-8%. PURs crosslink through a stepwise mechanism by reaction of the “end-blocking” isocyanate groups at the ends of the prepolymer backbone chains, first with water to form CO2 and amines, and then those amines with other isocyanate endblockers. Some PURs use latent hardeners such as ketamines or enamines that also form reactive amines by reaction with water, but without the CO2.
STPs crosslink by hydrolysis of alkoxysilane end-blockers at the end of prepolymer chains followed by condensation with alkoxy groups on other chains. The mercaptan-terminated polysulfide backbone molecules are typically joined by oxidative coupling with certain metal peroxides, such as PbO2. Typical concentration is 3-5%. The acrylic and rubber-based sealants cure by water or solvent evaporation, so there are no chemical crosslinkers.
Solvents (Including Water)
Low amounts of solvents, usually less than 5% by weight, are used in one-component PURs, STPs, and polysulfides to control viscosity and improve application properties such as extrusion, flow, and tooling. Typical solvents include xylene, heptane, acetone, mineral spirits, other alkylbenzenes, VP naphtha, hexane, methyl ethyl ketone, isopropanol, or deionized water. The water of the acrylic emulsions and solvents used in rubber-based sealants are critical to the actual formulation compounding. Concentrations range from 30-60%.
Adhesion Promoters
Included at about 0.5-3%, the most popular adhesion promoters are organofunctional silanes. These molecules have condensable alkoxysilanes at one end and a reactive functional group at the other. The former couples to the substrates being sealed; the latter is available for bonding to the sealant network itself. The functional groups may be mercapto-, epoxy-, primary and secondary amino-, glycido, or isocyanato-. The selection of one over another depends on the cure chemistry. Organic titanates are also used.
Antioxidants
Generally speaking, these compounds are typically not found in silicones, polysulfides or acrylics. They are used in PURs, STPs and rubber-based sealants at about 0.1-2%. For the most part, phenolic bases antioxidants are the norm. Commonly referred to as PAOs (phenolic antioxidants), these stabilizers work by reducing (deactivating) oxygen-centered radicals formed during the oxidation of organic compounds such as the polymer backbones.
PAOs are considered to be primary antioxidants because they are used to aid in long service durability. Secondary antioxidants, such as phosphites or thioethers, are seldom used in room-temperature, moisture-cure sealants.
UV Stabilizers
UV stabilizers—usually found in concentrations ranging from 1-8%—are critical in PUR, STP and rubber-based formulations, but are not usually found in silicone, polysulfide, or acrylic sealants. Two classes of stabilizers are typical:
• UV absorbers absorb UV light and convert the energy into molecular vibrations
• Hindered amine light stabilizers (HALS) work as a sort of highly efficient radical scavengers
UV absorbers can be organic or inorganic; the latter is usually referred to as a UV blocker. One of the best-known inorganic UV blocker is TiO2, though it is most often used for pigmentation and not as an absorber. The selection of a particular absorber or HALS and its concentration depends on the cure chemistry. For example, only very low levels of HALS are used in PUR due to the reaction potential of the amine group with isocyanate. Conversely, much higher loading is possible in STPs since these sealants do not contain isocyantes. HALS are the most effective for surface and bulk stabilization, whereas the absorbers do very little to protect the surface of a sealant.
Catalysts
Catalysts are a very small part of any formulation and are usually added at far less than a few tenths of a percent. Typical catalysts for PURs, STPs, and silicones are organometallic compounds, such as complexes of tin (Sn), titanium (Ti), or platinum (Pt). One of the most common catalysts is dibutyltin dilaurate (DBTDL). Some polysulfide formulations employ inorganic oxide activators and sulfur-based accelerators.
Other Components
Depending on the backbone and cure chemistry, formulations may also contain the following:
• Antimicrobials or biocides (all)
• Rheology modifiers (acrylics)
• Moisture scavengers (PUR, PS, STP )
• Surfactants (acrylics)
• Surface-drying regulators (acrylics)
• Pigments
It is apparent that sealant formulations contain many components that could, either separately or in concert with other components, result in discoloration under the right conditions of heat, light, stress, or intruding external contaminates.
Backbones
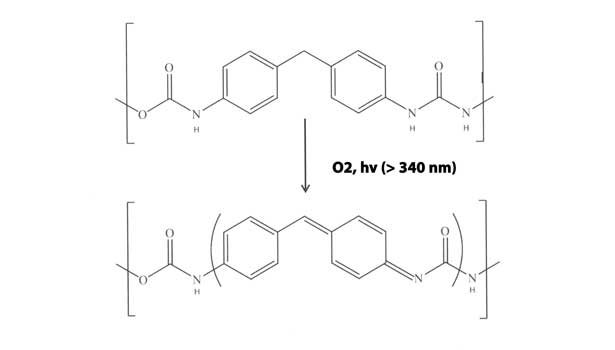
Of the six main types of sealants, single-component urethanes are arguably the most prone to backbone discoloration. This is most acute for those formulations based on aromatic isocyanate endblockers, particularly methylene 4,4-diphenyl diisocyanate (MDI). The susceptibility of this backbone to photooxidation is due to activation of the aromatic ring by absorption of UV light, subsequent oxidation of the central methylene unit and rearrangement to the chromophore.6 The simplified mechanism is shown in Figure 4. This mechanism is also consistent with the observed lower discoloration tendency of polyurethane sealants based on aliphatic endblockers (e.g., isophorone diisocyanate). In this case, there is no aromatic structure to absorb UV.
In addition, oxidation of polyether segments in these types of polyurethanes can lead to the formation of other functional groups, such as ahdehydes, as well as numerous radical species. These radicals can then induce the oxidation of the central carbon atom in the MDI. As a result, it is believed that formulations with long polyether segments are more prone to yellowing.7
Discoloration is not limited to PURs based on MDI; it can also be seen in those based on toluene diisocyanate (TDI). In this case, the visible light chromophore is a result of isomerization of the urethane or urea linkage to the enol form and conjugation with the aromatic ring.
Several different mechanisms have been proposed to explain the cracking, chalking and overall surface dulling that often accompanies weathering of PURs. Here, chain fragmentation results in formation of low-molecular-weight species as well as crosslinking that can lead to embrittlment and loss of flexibility. Depending on the endblockers used on the prepolymer chains, STPs based on alkoxysilane-terminated polyurethanes (sometimes called SPURs) can undergo the same discoloration mechanism as PURs. However, because of the typical higher loading of UV stabilizers, discoloration can sometimes be mitigated.
Acrylic polymers are generally known for their good outdoor weathering resistance; this is also the case for sealants made with such backbones. The polymers of acrylic sealants are primarily made up of alkyl acrylate or methacrylate repeat units, the –C-C- portion of which are quite stable against both photo- and thermooxidation. In addition, the ester carbonyls of the side groups generally have UV absorption maxima near or below the UVB range. However, as with many polymers, impurities incorporated during the polymerization process may absorb UV light, which could lead to the formation of visible light chromophores.
Some acrylic sealants use copolymers of acrylic esters with other monomers to enhance certain properties, such as solvent resistance or hydrophobicity.8 The co-monomers used (e.g., acrylonitrile, styrene or vinyl chloride) may undergo reactions that could result in discoloration.
Solvent-borne sealants based on styrene-butadiene-styrene (SBS) or styrene-isoprene-styrene (SIS) block copolymers are typically limited to indoor use, since these polymers are susceptible to photo- and thermooxidation. It is commonly believed that the oxidation starts at the unsaturated rubber portion and can lead to photodegradation of the polystyrene block, as well as any other copolymer units that can cause severe discoloration. Accordingly, in sealants intended for outdoor use, block copolymers with saturated rubber blocks are typically used.9 These include styrene-ethylene-butadiene-butadiene-styrene (SEBS) or styrene-ethylene-propylene-styrene (SEPS).
Sealants based on linear polysulfides generally show good weatherability, despite the low bond dissociation energies of the -S-S- and C-S- linkages and subsequent susceptibility to thermolysis and photolysis. The durability may be attributed to the fast recombination of radicals formed from either initiation route. Such recombination results in a stress relaxation of the polymers, but does not yield any visible light chromophores.
Mahon observed a yellow-brown discoloration of a MnO2-cured polysulfide sealant after 1,000-hour exposure to UV light.10 The discoloration was attributed to formate ester formation from photoinduced cleavage of the formal groups in the polymer chains, followed by reaction with oxygen. The author does not offer any details on the exact nature of the chromophore.
Most silicone sealants are based on poly(dimethylsiloxane) (PDMS) polymer. The –Si-O-Si- bond high dissociation energy (about 443-530 kJ/mol) and lack of any chromophores along the chains means outstanding resistance to heat and light and thus a low tendency to discolor. Arguably, these sealants are best-in-class in this regard. Because of this stability, silicone sealants are often formulated without UV stabilizers or antioxidants. Several mechanisms for the oxidation of the methyl side groups have been proposed, but the resulting products are claimed not to interfere with mechanical properties or cause discoloration.7,11
Fillers
Generally speaking, inorganic fillers are believed to essentially stabilize most sealant formulations against discoloration. Two cited mechanisms of filler stabilization are most noteworthy: interaction between filler and polymer backbone or other components, and reflecting and scattering or blocking of UV light.
The interaction of polymer with filler can limit conformational movement of the former, or in the case of strong adsorption, the polymer cannot assume its most preferred conformation. In these instances (especially the latter), the polymer is less susceptible to attack by UV light and hence less likely to discolor.
Inorganic fillers such as talc, calcium carbonate and titanium dioxide reflect UV light. Titanium dioxide and other pigment fillers have also been shown to absorb shortwave UV and convert it to heat. In this case, consideration should be given to the possibility of localized heating and its potential to initiate a mechanism to discoloration.
Conversely, fillers can directly or indirectly cause discoloration. In one example, titanium dioxide has been shown to play a major role in discoloration schemes with other sealant components. In another example, in the same way fillers can interact with the backbone polymer and retard discoloration, they can also adsorb components such as antioxidants or light stabilizers and thereby facilitate a color change. Iron oxide impurities found in some grades of talc can also interact with certain plasticizers, such as alkylsulphonic acid esters, and may cause them to yellow.
Plasticizers
The concern with plasticizers is often not how they might discolor a sealant, but rather how they might discolor the substrates being sealed. This is due to the fact that there can be some migration from the sealant.
In PURs and STPs—and to some extent polysulfides and acrylics—the phthalate esters such as diisodecylphthalate (DIDP), dioctylphthalate (DOP), and diisooctylphthalate (DIOP) are the norm. As a group, phthalate ester plasticizers have good thermal stability, but these types of molecules do contain functional groups that can absorb into the terrestrial UV range (280-400 nm) and are therefore susceptible to photooxidation, which could lead to the formation of radicals and ultimately visible light chromophores.12 In praxis, however, these types of plasticizers exhibit excellent resistance to outdoor discoloration, and any discoloration effects may arguably be due to the natural slight color of the plasticizer or impurities.13 One such impurity is unsaturations in the starting alcohols used in the manufacturing process (esterification of phthalic anhydride).
Many phthalate ester plasticizers have branch points in their aliphatic portions and hence labile tertiary hydrogens that could contribute to oxidation reactions that lead to the formation of chromophores in the chain or in other components. Alternatives to the phthalates include long-chain adipates and alkylsulfonic acid esters with phenol (ASE). These materials offer better hydrolysis resistance but are themselves yellow and can be made more so on contact with iron.14
The typical plasticizers in quality silicone sealants are known as silicone fluids, which are basically low-molecular-weight polysiloxanes. As with the backbone, these silicone fluids have outstanding resistance to heat and light and thus a low tendency to discolor. Lower-cost silicone formulations may contain high-boiling paraffin oils rather than silicone oils and may therefore show some discoloration over time.
Some polysulfides are formulated with chlorinated paraffin plasticizers, which depending on molecular weight and chlorine content, could discolor through dehydrochlorination.
Finally, plasticizers, or more specifically the incompatibility of such with the overall sealant formulation, can affect clarity. Gross incompatibility may manifest as a cloudy appearance, especially in clear or translucent formulations.
Solvents
Pure solvents do not lead to discoloration, but they can affect the color and intensity of chromophores. This effect in which compounds show different colors in different solvents is known as solvatochromism. Positive solvatochromism corresponds to a bathochromic (or red) shift, while negative solvatochromism corresponds to a hypsochromic (or blue) shift. In this context, the chromophore is called solvatochromic.
The type of shift depends on several factors, the most critical of which include the solvent’s polarity, the type of transition within the chromophore, whether the solvent interacts with the ground state or excited state of the chromophore, and solubility. For example, if a particular chromophore is soluble in a polar solvent, and that solvent lowers the energy of the excited state more so than that of the ground state, then light absorption would shift to longer wavelengths (lower energy). In other words, a red or bathochromic shift would occur. Depending on the solvating power and degree of interaction, the shift could be large enough to bring absorption into the visible light range. It should be noted that polarity effects on chromophores are not limited to those from solvents and could be extended to the entire sealant formulation or system.
One well-known solvatochromic substance is iodine. Elemental iodine (I2) is a black-blue solid at room temperature and violet-pink in the vapor phase. Solutions of iodine in non-polar solvents (e.g., hexane or napthas) are violet, since the energy difference between the ground and excited state is the same as in the gas phase. However, in a polar solvent such as ethanol, a charge transfer complex is formed and creates a brownish solution. In an aromatic solvent, such a xylene, the solution is a very dark red.
This solvatochromism is shown in Figure 5. A diiodo-based fungicide was dissolved in three different solvents (from left: naptha, ethanol and xylene) and exposed to sunlight.
Adhesion Promoters
Silane coupling agents are generally used in all sealant formulations. These molecules have reactive alkoxysilane groups at one end that essentially “couple” with the surfaces to be sealed. At the other end is a functional group that reacts with the sealant matrix. The most common functional groups are amines, epoxies, mercaptans and acrylics.
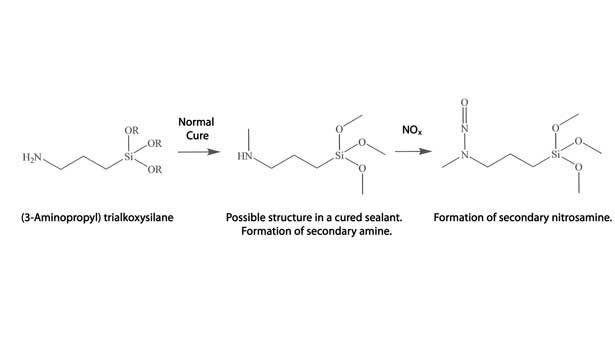
It is well known that the most popular type of promoters, namely the aminosilanes, are readily oxidized and will yellow. This discoloration may be the result of photooxidation of the amine group to form a nitrosamine.15 As mentioned earlier, a nitroso group is an independent chromophore.
Oxidation also proceeds with other oxidizing agents, and one in particular is NOx. Testing results showed discoloration of a one-component silicone sealant during exposure to both NO and NO2. The discoloration did diminish when the samples were exposed to sunlight over three days.
Discoloration of many sealant formulations by NOx is well known and has been shown to result from over-oxidation of phenolic antioxidants. However, silicone sealants generally do not contain antioxidants or UV stabilizers because of the stability of the polymer backbone against photo- and thermooxidation. So why would the silicone sealant yellow? One possible explanation is oxidation of the aminosilane adhesion promoter in the formulation. The end product would be the nitrosamine. A possible mechanism is offered in Figure 6. The mechanism is shown with a secondary amine since yield of nitrosamine is highest compared to primary and tertiary amines.
Antioxidants
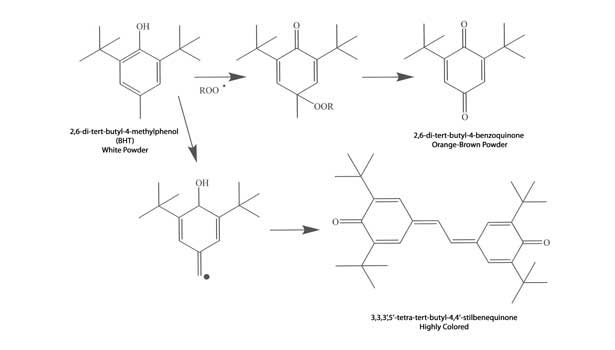
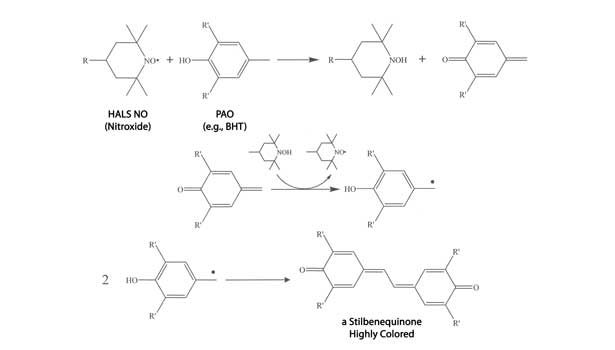
The commonly used phenolic antioxidants (PAOs) can discolor simply by performing their function—reducing oxygen-centered radicals—or via routes involving interactions with other sealant components, such as pigments or hindered amine light stabilizers (HALS). Mechanisms leading to discolored products from normal antioxidant function of the simplest PAO, namely BHT (2,6-tert-butyl-4-methylphenol), are shown in Figure 7. In this mechanism, the alky or aryl peroxy radical, ROO•, is generated from oxidation of other sealant components.
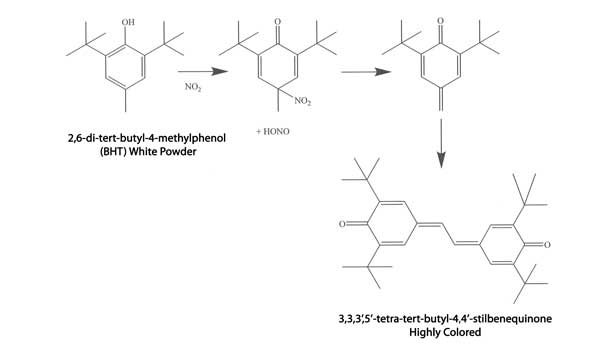
Another commonly encountered discoloration problem is from over-oxidation of the PAO by NOx. Such discoloration is sometimes referred to as “gas fading.” NOx gas is a mixture of several oxides of nitrogen and is formed during the combustion of fuels. Accordingly, discoloration of sealants can occur in areas with, for example, heavy forklift traffic or where gas heaters are being used. The UV light of a welding arc can also produce NOx from the nitrogen and oxygen in the air. A mechanism of NOx discoloration is shown in Figure 8.
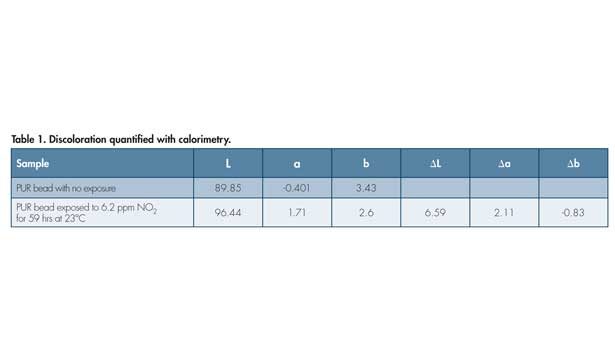
In one example of discoloration of a PUR from NOx, the PUR was based on aliphatic isocyanate endblockers; the PAO was not BHT, but rather a more complex, tetrafunctional molecule that is known to afford pink discoloration in aliphatic type plastics. In this example, the cured PUR sealant was exposed separately to NO and NO2, the two principal gases under the designation NOx. Discoloration was found to occur faster with NO and even at rather low levels of each (1 ppm or less). The discoloration of the pink samples was quantified with colorimetry. The CIELAB color and delta values are shown in Table 1. Note the positive a value and recall that this indicates a redder material after aging.
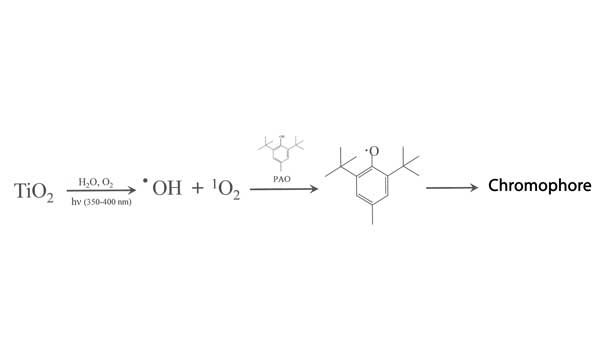
Over-oxidation of PAO can also occur from interaction with a common sealant pigment—TiO2 (titanium dioxide). The mechanism here is initiated by UV, which reduces the Ti+4 to Ti+3. The latter is highly reactive and causes the formation of hydroxyl radicals (•OH) and singlet oxygen (1O2) from water and oxygen. These later species can go on to oxidize PAOs to chromophores in much the same way as depicted in Figures 6 and 7.16 The mechanism is depicted in Figure 9. It should be noted that basic conditions facilitate this reactions, and that components such as HALS provide such an environment.
UV Stabilizers
The two types of organic UV stabilizers include UVAs (UV absorbers) and HALS (hindered amine light stabilizers). In reviewing the literature and from discussions with several sealant manufacturers, it seems that there are differences of opinion on the effectiveness of UVAs. Some manufacturers state that UVAs are crucial in their formulations, especially when combined with HALS; others say they are ineffective and not used.
The latter can be understood by considering the fact that, as true light absorbers, UVAs’ function follows Beer’s Law [absorption = (´) x (concentration) x path length]. This simple equation shows that UVAs are more effective in thick sections through a sealant, but not so much in thin sections or at the surface. HALS, on the other hand, are not UV absorbers, but rather highly efficient free-radical traps. Accordingly, their function is independent of thickness.
No matter the type, both UVAs and HALS may contain chromophores; depending on the exact structure, some are yellow in visible light. Note that both types may also have functional groups that can react with isocyanate endblockers. Therefore, if they are used, it is at a very low level. Conversely, since sealants such as STPs have no isocyanates, stabilizers are used at much higher levels.
The stabilizers’ functional groups, particularly the –OH of the UVA, much like the same group of a PAO, can interact with the –NH of HALS. The interaction can be synergistic (basically increasing the beneficial effects of each) or antagonistic (generally wasting the PAO and in some cases leading to a visible light chromophore or discoloration).
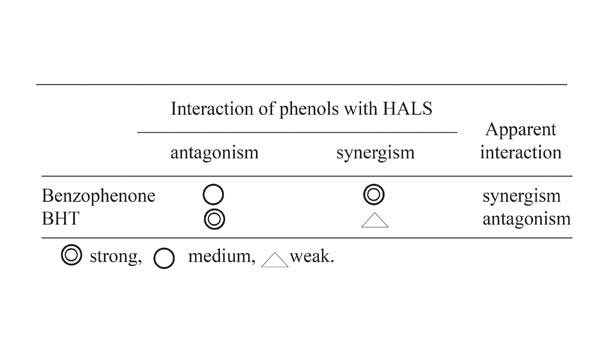
Such a comparison of benzophenone UVAs and BHT was made by Takenaka, et al.; results can be found in Figure 10.17 Ohkatsu and Fujiwara have studied the antagonistic interaction of PAO with HALS that leads to visible light chromophores and proposed a novel mechanism, as shown in Figure 11.18
Catalysts
Discoloration of organometallic catalysts is well known and is believed to be due to the chelation of the metal by other components such as UVAs or aminosilane adhesion promoters. Yellowing can also develop in systems that include 2,4-pentanedione in the presence of amines or silica. Tertiary amines are less likely to cause a problem than primary and secondary amines.
Other Factors and Effects
Many other effects can lead to the chemical discoloration of a sealant.
Microbial
Microbes are all around us, feeding on us and the things we make—including sealants. Microbes especially enjoy plasticizers, but nearly any organic material can be food. Generally, microbial discoloration produces the familiar black or green associated with mold, but other colors are possible depending on the species. Microbial discoloration usually appears as spots and is not continuous over the surface.
Antimicrobial
These substances kill or inhibit the growth of microorganisms such as bacteria, fungi, or protozoans. Obviously, these will reduce discoloration by microbes, but some antimicrobials can themselves cause discoloration. One group in particular includes those with covalently bonded iodine. These popular antimicrobials are highly effective but are concurrently susceptible to photooxidation and will decompose to yield elemental iodine and free radical components that appear yellow to brown in color.
A good example of this effect is shown in Figure 12. Here, a translucent silicone containing a diiodoalkyl-tolylsulfone antimicrobial showed no color after cure and through two weeks at 60ºC or one day at 110ºC. However, when placed in direct sunlight, the pink color appeared within one hour, but disappeared shortly thereafter. It is believed that elemental iodine was liberated by the terrestrial UV and subsequently diffused through the silicone to sublime. It must be noted that the exact mechanism causing the discoloration was not fully elucidated.
Wood Lignins and Formaldehyde
Wood and wood-based products contain numerous chemicals; of most interest in the context of this discussion are lignins, various intrinsic chromospheres, and formaldehyde. A sealant in direct contact with certain woods might absorb some of the natural chromophores and discolor. Formaldehyde, the principal VOC in many wood-based products, may also contribute to discoloration, but no mechanisms could be found.
Figure 13 shows an originally white STP sealant used on RV rooftops. The part shown is a display piece and was never exposed to UV or heat. It was shipped in a plywood container and stored there for several months. It is believed that lignins or formaldehyde in the wood or wood adhesive are responsible for the discoloration.
Welding and O3 Generation
Ozone (O3) is produced by UV light from the welding arc. Ozone is a substantially strong oxidizer, more so than regular, diatomic O2, and high concentrations in and around curing or cured sealants could cause fast oxidation of the surface.
For additional information, contact Sika Corp. at (248) 577-0020 or visit www.sikausa.com.
References
1. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”), compiled by McNaught, A.D., and Wilkinson, A., Blackwell Scientific Publications, Oxford, 1997; XML online corrected version: http://goldbook.iupac.org, created by Nic, M., Jirat, J., Kosata, B.; updates compiled by Jenkins, A., 2006, ISBN 0-9678550-9-8. doi:10.1351/goldbook.
2. Kaurav, M.S., Engineering Chemistry with Laboratory Experiments, PHI Learning Private Ltd., New Delhi, India, 2011, pp. 265-266.
3. Commission internationale de l'éclairage, The International Commission on Illumination.
4. “CIE L*a*b* Color Scale,” Hunter Lab Application Notes, Insight on Color, Vol. 8. No. 7, Hunter Lab, 2008, www.hunterlab.com/appnotes/an0198r.pdf, accessed April 12, 2012.
5. “The ASTM D 6166 Gardner Color Index,” Hunter Lab Application Notes, Insight on Color, Vol. 10. No. 1, 2008, www.hunterlab.com/appnotes/an0198r.pdf, accessed July 28, 2012.
6. Wypych, George, Handbook of Material Weathering, 2nd Edition, ChemTec Publishing, Ontario Canada, 1990, pp. 275-396.
7. Wolf, Andreas, “Construction Sealants,” Handbook of Sealant Technology, Chapter 13, Mittal, K.L. and Pizzi, A., editors, CRC Press, Taylor and Francis Group, LLC, Boca Raton, FL, 2009, pp. 311-378.
8. Petrie, Edward M., “Sealants Based on Acrylic Emulsions,” SpecialChem, April 5, 2006, www.specialchem4adhesives.com/resources/articles/article.aspx?id=1538, accessed August 2, 2012.
9. Petrie, Edward M., “Styrene Butadiene Block Copolymers as Solvent Borne Sealants,” SpecialChem, March 21, 2012, www.specialchem4adhesives.com/resources/articles/article.aspx?id=5715#, accessed September 25, 2012.
10. Mahon, A., Linear Polysulfides: Their Characterization and Degradation Pathways, Ph.D. Thesis, University of Warwick, Warwick, England, 1996.
11. Tomer, Namrata S., Delor-Jestin, Florence, Frezet, Lawrence, Lacoste, Jacques, “Oxidation, Chain Scission and Cross-Linking Studies of Polysiloxanes upon Ageings,” Open Journal of Organic Polymer Materials, 2012, 2, 13-22.
12. Lyman, W.J., et al; Handbook of Chemical Property Estimation Methods., Amer Chem Soc. Washington, DC, 1990, pp. 8-12.
13. “Improving the Color of Plasticizer Esters,” VenPure® Polisher, Rohm & Haas Co., July 2003, www.rohmhaas.com/assets/attachments/information/industries/industrial/chem_processing/VenPure_Polisher_plasticizer_esters.pdf, accessed August 3, 2012.
14. Messomol® Technical Information Sheet, LANXESS Deutschland GmbH, Edition 2010-10-15.
15. Pitts, J.N., et al., “Photooxidation of Aliphatic Amines Under Simulated Atmospheric Conditions: Formation of Nitrosamines, Nitramines, Amides, and Photochemical Oxidant,” Environ. Sci. Technol., 12(8), 946-953, 1978.
16. Scheirs J., Compositional and Failure Analysis of Polymers. A Practical Approach, John Wiley & Sons, NY, 2000, p. 709.
17. Takenaka, H., Mizokawa, S.,Ohkatsu, Y., “Interaction of Hindered Amine Light Stabilizers and Ultraviolet Absorbers,” Journal of the Japan Petroleum Institute, 50 (1), 8-15, 2007.
18. Ohkatsu, Y., Fujiwara, T., “Interaction between Nitroxide of Hindered Amine Light Stabilizers and Phenol,” Journal of the Japan Petroleum Institute, 50 (2), 87-93, 2007.