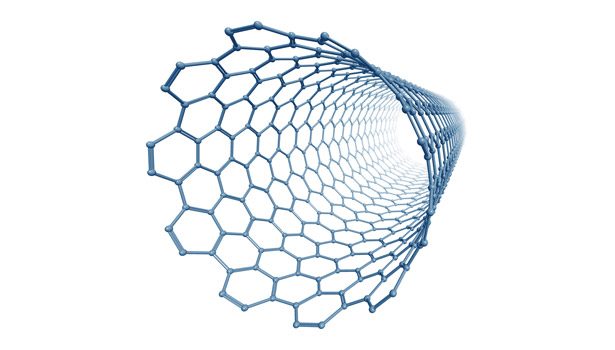
Carbon nanotubes (CNTs) are nanometer-scale tubular structures consisting entirely of carbon atoms (see Figure 1). They represent a newly discovered elemental form of carbon and have remarkable properties in terms of mechanical strength, thermal conductance and electrical conductivity. CNTs are the strongest materials ever discovered, with tensile strengths reported to be 50 times that of steel. They can also have conductivities 1,000 times that of copper.
Since their discovery, CNTs have been an intense focus area in nanotechnology research. Advancements in synthesis technologies have now allowed for the large-scale production of CNTs such that these materials are finding their way into commercial product applications.
CNTs are available in a variety of forms, including single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). In addition, MWNTs can be produced having a “Russian doll” structure consisting of individual tubes within tubes, or having a “parchment” structure consisting of a continuous, multi-rolled sheet of carbon. CNTs can also come in a variety of chiralities based on how the sheet of carbon atoms are connected together (including so-called “zig-zag” and “armchair” configurations), as well as in a range of different sizes, with typical diameters of 1-3 nm and lengths that approach several microns (with aspect ratios exceeding 1,000x).
Different types of CNTs can exhibit large differences in terms of strength, conductivity and other properties. Commercially available CNTs normally consist of complex mixtures of tube types and sizes, thus making proper CNT selection a critical requirement for successful results in a given application.
Use of Carbon Nanotubes in Corrosion-Control Coatings
A new series of corrosion-control coatings containing CNTs is now being developed and made available for end-user sampling. These coatings have enhanced strength and electrical conductivity due to the inclusion of CNTs, while also incorporating sacrificial metal particles (e.g., Zn, Al, Mg) for corrosion inhibition via cathodic protection. These coatings can be applied using standard painting equipment (conventional and HVLP air spray, airless spray, brush, roller, etc.) and are based on traditional coating polymers, including epoxy and urethane chemistries. CNTs allow sacrificial metal-filled primers to be formulated at much reduced metal content vs. traditional systems.
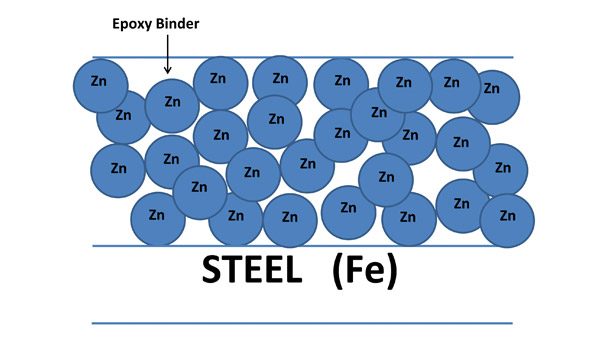
Although epoxy primers filled with zinc dust (e.g., MIL-PRF-24441/19) have been widely used for heavy-duty industrial maintenance applications for many years, these primers (also known as zinc-rich primers) must be formulated with very high levels of zinc dust (80-90% wt) to ensure point-to-point contact of zinc particles and effective galvanic activity (see Figure 2). As such, these coatings can be brittle/porous and exhibit poor substrate and/or inter-coat adhesion due to the binder-starved condition of the coating film. The application of an intermediate coating is normally required to stabilize the primer film before topcoat application.
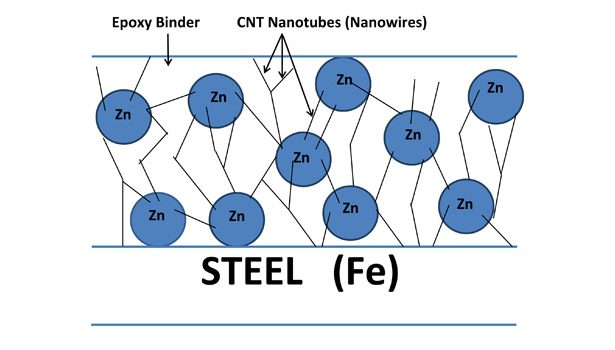
Alternatively, in a CNT/Zn-based epoxy primer, CNTs are used to establish conductive pathways within a coating film so that lower levels of zinc metal are required (see Figure 3). This results in a tougher, more binder-rich primer film that exhibits better flexibility and adhesion. An intermediate coating is not required before final topcoat application. The use of a two-coat, CNT-based system can thus provide considerable project cost/time savings vs. conventional three-coat systems.
The establishment of conductive networks in the coating film occurs due to the unique ability of carbon nanotubes to bond together and form rope-like structures. Strong van der Waals interactions occur between CNTs. They can also bond together covalently through a process known as p-stacking.
Field Testing of CNT/Epoxy Zinc-Rich Coatings
In 2009, a CNT-based epoxy primer containing zinc dust was demonstrated in a field trial with the U.S. Army Corp of Engineers at Fort Bragg, N.C. In this project, a CNT/Zn-based epoxy primer/urethane coating system was applied to the exterior of a steel storage tank that was suffering from corrosion damage.
The existing coating, a conventional three-coat zinc-rich epoxy/epoxy intermediate/urethane topcoat system, was removed with abrasive blasting to SSPC SP10 near-white metal specifications, followed by the application of the two-coat CNT-based system. Extended immersion, salt-fog and outdoor fence testing of the coatings was also conducted. Results have been excellent, and the U.S. Army Corp is considering possible implementation of the coatings as a replacement for the traditional three-coat, mil-spec systems.
A Technology Platform for Advanced Coatings
The excellent results obtained with CNT/Zn-based systems during field and laboratory testing with the U.S. Army Corp show that these coatings may offer an important technology platform for enhanced corrosion-protective systems in other coating applications beyond the atmospheric protection of steel substrates. For example, other types of coatings can be developed and tailored for different applications through the careful selection of CNT types, polymer chemistries, and sacrificial metal choice.
For example, research is now being conducted on CNT-based coatings containing sacrificial magnesium pigments for the protection of aluminum substrates in aerospace applications. Magnesium can provide cathodic protection to aluminum in the same way that zinc provides protection to steel, and significantly reduced levels of magnesium are needed when conductive CNTs are incorporated.
Current aerospace primers rely on a passivating mechanism to control corrosion by incorporating hexavalent chromate pigments that are highly carcinogenic and environmentally unsafe. A CNT-based coating using sacrificial magnesium would operate by a completely different mechanism and would not require chromate pigments. Hazardous pretreatment processes could be potentially eliminated as well.
Given the severity of corrosion problems in the U.S. and abroad, and the need for improved coatings, CNT-based coatings for corrosion-control applications should generate significant interest within the industry. The direct annual costs of corrosion in the U.S. are over $400 billion per year, and CNT-based coatings may represent an important new technology to help reduce costs.
For additional information, visit www.teslanano.com.