The photo curing extent of styrenic block copolymer (SBC)-based adhesive formulations applied as a uniform layer on a Mylar film can be readily determined by Fourier transform infrared spectroscopy (FTIR). Recently, a pulsed KrF excimer laser (emission line at 248 nm, pulse width ~30 ns) was incorporated as the UV source. The adhesive formulation prepared without the addition of a photoinitiator was exposed to the UV laser beam for various durations up to 3 µs (100 laser pulses) in order to cure the adhesive.
The absorption band at 910 cm-1 (vinyl groups) was followed to evaluate the photo-curing process. The determination of percent cure is an important parameter to optimize both the formulation manufacturing process and the adhesive performance.
The introduction of this kind of source opens up new possibilities for the initiation of the photo-crosslinking of SBC-based adhesives. This is an elegant way to obtain UV-curable adhesives without the addition of a photoinitiator.
Common Challenges
During the photo-curing of polymeric materials, coupling a maximum of the lamp energy into the material is challenging due to the different spectral regions where the lamps emit and the materials absorb.1 However, the more energy coupled, the more efficient the photo-curing process will be. Consider the example in Figure 1: The UV exposition of an adhesive formulation based on an SBC elastomer (e.g., S-1205) with a conventional Hg lamp. In cases where the elastomer absorbs far away (190-275 nm) from the region where the lamp emits its maximum energy, photoinitiators exhibiting absorption bands in the region 250-450 nm must be used.
Despite the use of photoinitiators, Figure 1 shows that a considerable amount of the lamp’s energy may be lost because its emission lies outside the absorption bands of the photoinitiator. This energy loss has adverse consequences in the overall photo-curing process. First, the process is not very efficient, so the treated material has to be irradiated for longer periods to achieve the desired extend of photo-curing. Moreover, part of the lost energy could be absorbed through phonon interactions by the polymer lattice; this increase in the material temperature can lead to it softening or degradation. All these disadvantages limit the photo-curing extent of the processed material.
Laser Benefits
In the continuous search for faster photo-processing, lasers may provide the ultimate light source to achieve ultra-fast polymerization and micronic resolution, due to the sharp focus and great power concentrated in the collimated laser beam. Laser-induced polymerization has been applied to different types of multifunctional monomers and oligomers (e.g., acrylates, vinyl ethers, epoxides) to produce highly crosslinked polymer materials.2-4 This advanced technology has found new applications in various industrial sectors where the improved performance has outweighed the higher cost of laser processing, particularly in photolithography, stereo lithography and holography.
Several features of laser radiation make it unique for applications in the photo-processing of adhesive formulations. Lasers emit in a narrow (a few nm) wavelength interval, which concentrates the total energy irradiated from this light source. That’s why the laser intensity (photon flux) in this wavelength interval is orders of magnitude higher than the intensity exhibited from other light sources. The laser emission wavelength can be carefully selected to fit the absorption band of the adhesive, with the consequent increase in the efficiency of the photochemical reactions considered.
Laser radiation is distinguished from other light sources through its spatial coherence, which means that all photons present in a laser beam own the same phase and direction.5 This characteristic enables a great directivity so that the laser beam can be focused down to a micrometric spot. Laser radiation is also temporally coherent, which reduces the extent of undesirable secondary reactions induced by polychromatic wavelength.6 By irradiating several types of composites with a 468 nm pulsed laser, Tark et al. hypothesized that due to the monocromaticity and coherence of a pulsed laser light, the attenuation will be lower; consequently, the radiation will penetrate deeper in the composite, resulting in a greater extent of monomer conversion.7
Lasers emit energy either as continuous wave (CW) or pulsed lasers. CW lasers emit energy continuously during the time they are switched on; pulsed lasers concentrate the total energy in short time intervals (pulses), which range in duration from milliseconds (ms, 10-3 s) to femtoseconds (fs, 10-15 s).
The short pulse width (τ) exhibited by pulsed lasers leads to the possibility of obtaining high peak powers (Epulse/τ), and consequently high power densities (Pulsepower/Spotarea), which can considerably increase the overall rate of the photochemical processes that occurs during the photo-curing. Particularly when using ns or fs laser pulses, the power density (fluence) can be so high that the damage threshold of the material could be exceeded. In such cases, a preliminary experimentation is mandatory in order to obtain the proper laser fluence (below the damage threshold) for the efficient photo-curing process.
In addition, material processing with ultra-short laser pulses can induce ultra-fast chemical reactions in photosensitive materials, which can then reduce the sample’s exposure time. For example, if a determined material sample is photo-cured with 100 laser pulses, each with a 30 ns pulse width, the total irradiation time will be 3 as (3 x 10-6 s), a value well below the typical time needed to photo-cure adhesive formulations with conventional polychromatic light sources—which lies in the order of seconds. This significant reduction of the irradiation times opens the possibility for the treatment of considerably greater areas than those covered with conventional polychromatic sources, with a properly scanning of the laser beam over the material surface.
Pulsed lasers can provide pulse widths in the order of nanoseconds and a repetition rate of several Hz. This exhibits another important feature that significantly differentiates this method from continuous wave laser curing.5 The ns pulses are very short compared with the period between laser pulses (tens of ms), which allows for material cooling between the laser pulses and consequently avoids material degradation.
As mentioned previously, photoinitiators are required to enable the photo-curing process in many cases. However, they have several drawbacks concerning the final product, such as a reduction of the photoinitiator concentration or even the possibility of photo-curing without photoinitiator. A reduction of the sample exposure time with an increase in the photo-curing extent of the adhesive formulation are features of great interest from the technical and commercial point of view.
Experimental Details
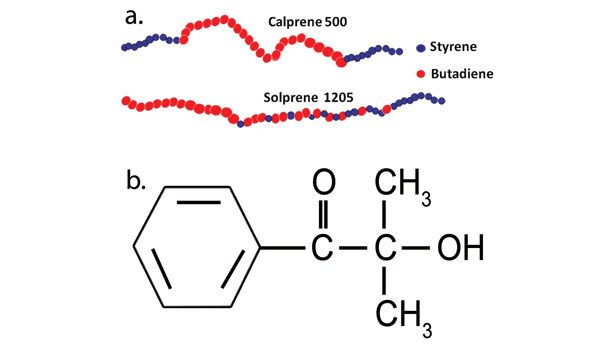
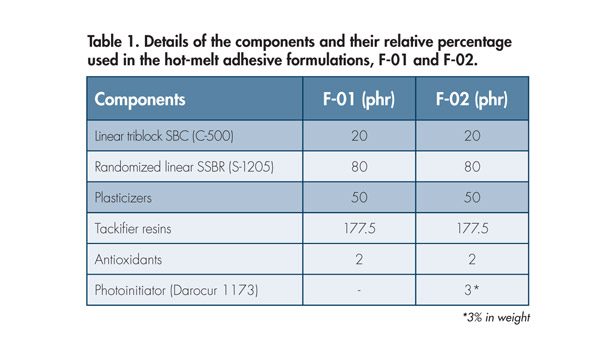
A polystyrene-block-polybutadiene-block-polystyrene (SBC) and a random copolymer styrene butadiene rubber (SSBR) were used in this study.* The materials also differ by their content of pendent vinyl groups: ~12-14% of the total double bond content for the SBC and 9% for the SSBR. Darocur® 1173 from BASF was introduced in the formulation as a photoinitiator, at a typical concentration of 3 wt%. The chemical structures are shown in Figure 2. Naphthenic/paraffinic oil was selected as plasticizer. Two types of stabilizers were introduced in the formulations, each at a typical concentration of 1 part per 100 parts rubber (phr).
The coated samples were made in a constant temperature/constant humidity-controlled room (23 ± 2°C, 50 ± 2% relative humidity) using a ChemInstruments hot-melt coater and adhesion release tester AR-1000. The typical temperature setting for the hot-melt coater was ~140°C for the top and bottom bars. The adhesive was coated at ~ 0.005 in. thickness on a face stock such as 2 mm PET film.
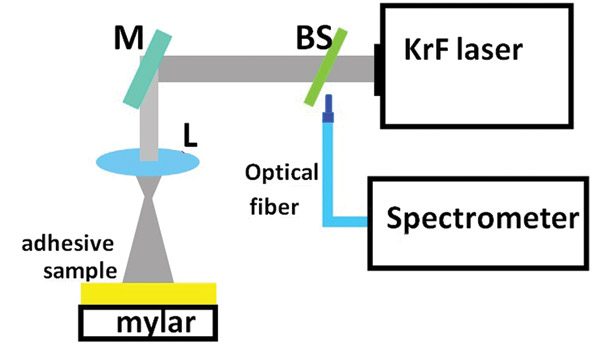
The experimental setup used to irradiate the samples is shown in Figure 3. The UV laser beam was directed to the sample using the mirror (M). Initially, the fluency at the target surface was of 0.6 x 106 W/cm2, which caused irreversible damage on the sample. In order to diminish the laser fluence at the sample surface, a convergent lens (L) was inserted in the beam path. The resulting spot was larger, covering an irradiation area of 13 cm2 of the sample; consequently, the power density at the sample surface was one-fourth (0.15 x 106 W/cm2) of the previous value, well below the damage threshold.
To collect the laser emission spectrum, a beam splitter (BS) was inserted in the beam path. The 4% of the laser beam reflected by the BS was fed through an optical fiber into an USB spectrometer to obtain the laser emission spectrum (see Figure 1). Table 1 presents relevant information about the components and the composition used to prepare each one of the two formulations investigated during the study.
FTIR Measurements
The first series of experiments was conducted in order to find the adequate fluence (below the damage threshold) for the samples irradiation. A laser fluence of 0.15 x 106 W/cm2
measured at the sample surface was found to be appropriate to conduct the rest of the experiments.
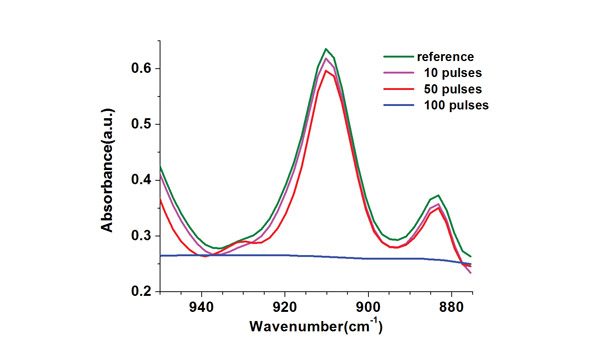
Figure 4 presents the FTIR spectra of the adhesive formulation F-01 (without photoinitiator), which was irradiated in the presence of oxygen with different laser pulse amounts. Results reveal the decrease of the absorption peak at 910 cm-1, corresponding to the S-1205 and C-500 vinyl groups (-CH=CH2) as photocrosslinking proceeds by increasing the number of laser pulses. The peak completely disappeared when the sample was irradiated with 100 laser pulses. A similar behavior has been reported by Scherzer et al. on the direct initiation of the photo polymerization of acrylates by using the 222 nm emission of a KrCl* excimer lamp.8 For this one, the most promising results have been obtained with acrylates containing ethoxy groups or chains. The addition of antioxidant blends in these formulations could have reduced the reaction of oxygen with the free radicals, enabling the photo-curing despite the air exposure.9
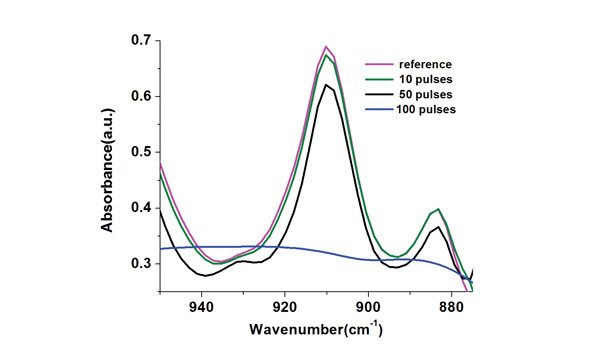
To study the influence of the photoinitiator in the photo-curing of these adhesive formulations, a second series of samples was prepared containing 3% Darocur 1173. In this case, the spectra were acquired from samples irradiated with 10, 50 and 100 pulses, respectively; results are presented in Figure 5. Upon UV irradiation, this formulation was not found to polymerize faster than the photoinitiator-free formulation since a very similar decrease of the absorbance peak (measured at 910 cm-1 by FTIR) was recorded for the samples. This shows that the photo-induced initiation reaction is already reaching its maximum efficiency in the neat formulation.
Successful Results
Several conclusions can be drawn following a comparison of the FTIR spectra obtained for the adhesive formulations based on SBS and SSBR elastomers. The use of a 248 nm excimer laser is feasible for elastomer photo-curing. An exposure time of 3 µs was enough to reduce the absorption peak at 910 cm-1, corresponding to the vinyl groups. This fact indicates that the curing process underwent a total conversion.
The complete disappearance of the vinyl band was obtained in the absence of a photoinitiator, and no remarkable improvement was found when the photoinitiator was added to the formulation. Thus, the process can reduce the costs of the formulation. The presence of oxygen did not affect the crosslinking reaction under the conditions studied. Since formulations were 100% solid (i.e., solvent free), this technology can be considered green and friendly to the environment.
For additional information, visit www.dynasol.com.mx.
References
1. Simbürger, H., Kern, W., Hummel, K., Hagg, C., “Photoreactions in Polymers Containing Benzyl Units: A Comparative Study under Excimer Laser and Hg-Lamp Irradiation,” Polymer, 41, 7883-7897, 2000.
2. Serbin, J., Egbert, A., Ostendorf, A., Chichkov, B.N., Houbertz, R., Domann, G., Schulz, J., Cronauer, C., Fröhlich, L., Popall, M., “Femtosecond Laser-Induced Two-Photon Polymerization of Inorganic-Organic Hybrid Materials for Applications in Photonics,” Optics Letters, 28, No. 5, 301-303, 2003.
3. Sun, X., Yin, D., Dai, H., Liu, J., Lu, R., Wu, S.T., “Intermittent Curing and its Effect on Pulsed Laser-Induced Photo Polymerization,” Appl. Phys., B 92, 93-98, 2008.
4. Lee, S.W., Park, J.W., Kwon, Y.E., Kim, S., Kim, H.J., Kim, E.A., Woo, H.S., Swiderska, J., “Optical Properties and UV-Curing Behaviors of Optically Clear Semi-Interpenetrated Structured Acrylic Pressure Sensitive Adhesives,” International Journal of Adhesion & Adhesives, 38, 5-10, 2012.
5. Houbertz, R., Steenhusen, S., Stiche, T., Sext, G., “Two-Photon Polymerization of Inorganic-Organic Hybrid Polymers as Scalable Technology using Ultra-Short Laser Pulses,” Coherence and Ultra Short Pulse Laser Emission, F. J. Duarte (Ed.), ISBN: 978-953-307-242-5, InTech, 2010.
6. Bosch, P., Fernández-Arizpe, A., Mateo, J.L., Corrales, T., Peinado, C., “New Fluorescent Probes for Monitoring the Polymerization Reaction Part 3: Pulsed-Laser Polymerization of Acrylic Adhesives,” Journal of Photochemistry and Photobiology A: Chemistry, 167, 229-236, 2004.
7. Tark, Z., Meniga, A., Ristie, M., Siitalo, J., Pichler, G., “Polymerization of Composites Using Pulsed Laser,” Eur. J. Oral Sci., 103, 394-398, 1995.
8. Knolle, W., Scherzer, T., Naumov, S., Mehnert, R., Direct (222 nm) Photopolymerization of Acrylates; A Laser Flash Photolysis and Quantum Chemical Study,” Radiation Physics and Chemistry, 67, 341-345, 2003.
9. Johnstone, J., Pressure Sensitive Adhesive Tapes, Chapter 4, Pressure Sensitive Tape Council, 2003.