Pure monomer resins (PMRs) are produced from pure aromatic monomer streams, especially styrene and alkyl styrene derivatives. Major advantages of PMRs, compared to other aromatic crude C-9 resins, include light color (water white), excellent thermal stability, temperature resistance (similar to hydrogenated hydrocarbon resins) and controlled molecular weight.1-3
PMRs are typically manufactured using Lewis acids/Friedel-Crafts catalysis of styrenic/alkyl styrenic monomer. Pennsylvania Industrial Chemical Corp./Hercules pioneered the process in early 1960s.4-7 Since then, several PMRs have been commercialized with varying vinyl aromatic chemistries and properties (softening points, molecular weight, etc.).
Using a proprietary processing technology, researchers recently developed several new PMRs that have very low residual monomer content and total volatile organic compound (VOC) content compared to traditional PMRs in the market. These new resins are based on a pure monomer resin product line that has been used for many years to modify styrenic block copolymers (SBCs), ethylene vinyl acetates (EVAs), and other polymers to balance cohesion, melt viscosity, and mechanical properties for various applications.8-15 This study is focused on evaluating the volatility characteristics of new low-volatile PMRs and their adhesive performance, especially in styrene isoprene styrene (SIS)-based pressure-sensitive adhesive (PSA) tape formulations.
Results and Discussion
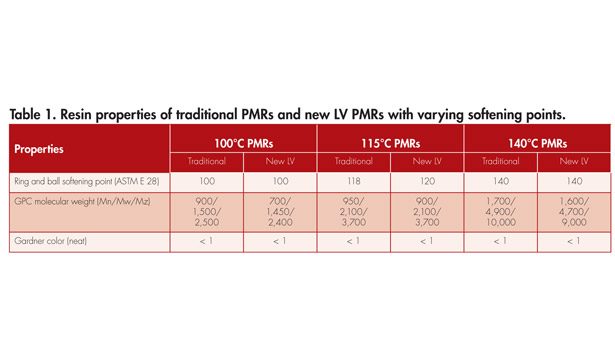
Resin properties of the low-volatile (LV) PMRs and traditional PMRs are given in Table 1. New LV PMRs show similar resin property characteristics as the traditional PMRs.
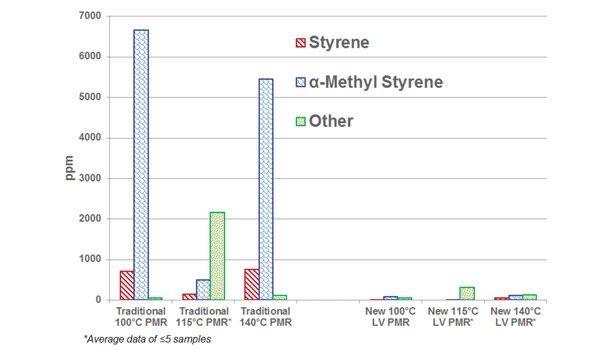
Residual monomer content and total VOC content of both traditional and new LV PMRs were analyzed using high-performance liquid chromatography (HPLC). HPLC has been employed by many in the industry to study the residual styrene content in several different applications.16 HPLC analysis was performed using Thermo-Fisher Hypersil gold column with an isocratic mobile phase. The peak area (UV wavelength monitored is at 200/210 nm), response factor (from 1-500 ppm), and sample weight were used to calculate the concentration of residual monomers and residual solvent in the resin sample as parts per million (ppm). HPLC analysis of the new low-volatile PMRs shows very low residual monomer content and total VOC content (other) compared to traditional PMRs available in the market (see Figures 1 and 2).
As shown in Figures 1 and 2, it is clear that LV grades of these PMRs show significantly lower residual monomer content and total VOC content compared to the traditional offerings in the market. It should also be noted that the variation in total VOC content of new LV PMRs is very low compared to the traditional PMRs.
The thermal stability of traditional and LV PMRs was analyzed by thermo-gravimetric analysis (TGA) at isothermal temperatures, using 15 mg of sample with a heating rate of 10°C/min and nitrogen purge rate of 80 ml/min. Weight loss was measured at every four seconds, and the percentage of weight loss was plotted against the isothermal time (see Figure 3). The LV PMRs show lower weight loss over time at all temperatures due to reduced total VOC content, resulting in better thermal stability. Improved thermal stability would lead to improved high-temperature storage and processing stability.
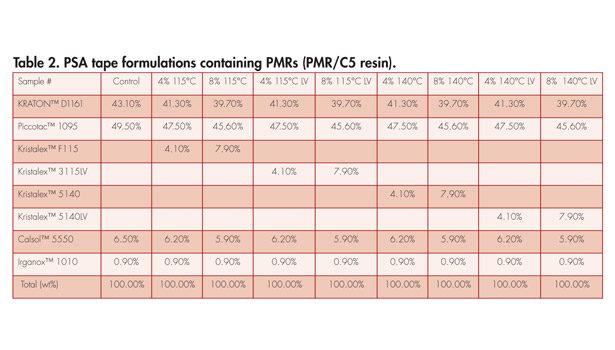
These new LV PMRs were also evaluated in an SIS-based hot-melt tape formulation to understand the adhesive performance characteristics in comparison to traditional PMRs. Table 2 shows the hot-melt tape formulations used in this study. These formulations used a midblock compatible resin and an endblock reinforcing PMR. This type of formulation is well-known in the industry where PMRs are used as a styrene block reinforcing agent9 in an SIS polymer to improve the melt viscosity characteristics and cohesive strength8,9 along with a midblock compatible C5 resin (Piccotac™ 1095). Two PMR addition levels (4 and 8 wt%) were evaluated to understand the concentration effect on adhesive properties and viscoelastic characteristics, especially melt flow.
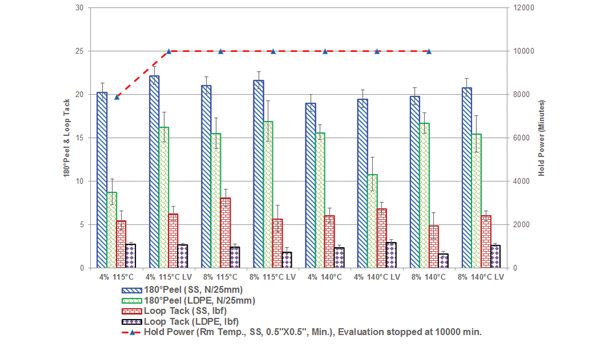
Adhesive blends were processed using a Plasticorder Brabender at 150°C/100 rpm with roller blades. The polymer was initially masticated for several minutes before the resin was added. Solid resins were premixed and then added to the polymer and blended for 20 minutes. Oil was then introduced, and the formulation was mixed for additional 10 minutes. Adhesive blends were direct coated onto Mylar (2 mil) using a hot knife coater at 150°C to achieve a coat weight of 20-25 g/m2. Tape was then cut into 1-in. strips for testing. The effect of adhesive peel and loop tack (PSTC-101 and PSTC-16)17 was evaluated on two different substrates with varying surface energies (stainless steel and LDPE), and shear strength/holding power (modified PSTC-107)17 was evaluated on stainless steel. The results are shown in Figure 4.
As shown in Figure 4, no significant differences in adhesive performance characteristics (peel and loop tack) were observed for the LV PMR-containing formulation compared to the non-LV counterparts on both high-energy (stainless steel) and low-energy (LDPE) substrates. At higher PMR addition levels (8%), higher hold power values were obtained; this is believed to be mainly due to effective reinforcement of styrenic phase.8,9
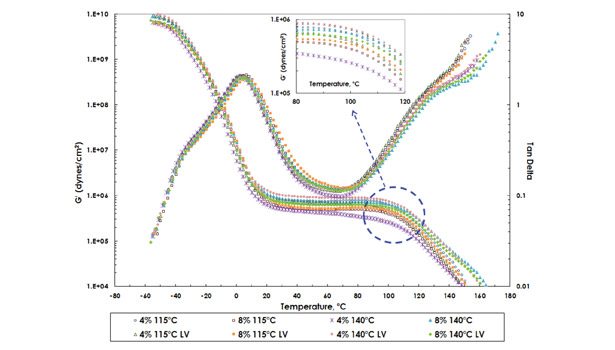
Viscoelastic characteristics of PMR-containing formulations were evaluated using dynamic mechanical analysis (DMA), as shown in Figure 5. A TA Instruments Ares RDA3 Rheometer was used in a parallel plate geometry (8 mm plate, 1.704 mm gap) and autostrain mode (maximum strain at 5%) at 10 Hz frequency with a heating rate of 6°C/min. Figure 5 shows no significant differences in Tg, and the elastic modulus (G’ at room temperature) and melt flow temperatures were observed between traditional PMRs and LV PMRs. These correlate well to the adhesive performance characteristics.
A Sustainable Alternative
It is evident that new low-volatile (LV) grades of PMRs exhibit significantly lower residual monomer content and total VOC content compared to the traditional offerings in the market. New LV PMRs also show less variation in total VOCs and improved thermal stability characteristics.
PSA tape evaluation of LV PMRs showed similar cohesive (shear/hold power) and adhesive performance characteristics (peel and tack) to traditional PMR-based PSAs. The new LV PMRs can be used to modify SBCs, EVAs, and other polymers to balance cohesion, melt viscosity, and mechanical properties while now also providing the low VOC levels (< 500 ppm) needed for today’s environment.
For additional information, call (800) 327-8626, email adhesives@eastman.com or visit www.tackifier.com.
Acknowledgements
The authors would like to thank Jennifer Morton, Courtney Saracco, Timothy Williams, Andrea Hagood and Deborah Moroney of Eastman Chemical Co. for their support in obtaining data.
References
1. Holohan Jr., J.F., Penn, J.Y., Vredenburgh, W.A., Kirk-Othmer Encyclopedia of Chemical Technology; Wiley InterScience, New York, 1980, 3rd Ed., Vol. 12, pp. 852-869.
2. Collin, G., Mildenberg, R., Zander, M., Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag Gmbh & Co., Weinheim, 2003, 6th Ed., Vol. 31, pp. 357-369.
3. Findlay, J., Bikales, N.M., Encyclopedia of Polymer Science and Technology; John Wiley & Sons, New York, 1968, Vol. 9, pp. 853.
4. Powers, P.O., U.S. patent number 3000868, 1961.
5. Campbell, C.C., Finfinger, D.A., U.S. patent number 3630981, 1971.
6. Douglas, P.S., Patellis, A.P., Vredenburgh, W.A., U.S. patent number 3932332, 1976.
7. Campbell, C.C., Finfinger, D.A., U.S. patent number 3956250, 1976.
8. Class, J.B., Chu, S.G., J. Appl. Polym. Sci., 1985, 30, 805.
9. Class, J.B., Rubber Chemistry and Technology, 1985, 58, 973.
10. Douglas, P.S., Patellis, A.P., Vredenburgh, W.A., U.S. patent number 4075404, 1978.
11. Douglas, P.S., Patellis, A.P., Vredenburgh, W.A., U.S. patent number 4113801, 1978.
12. Carper, J.D., Tappi Journal, June 1990, 167.
13. Dunckley, P.M., Adhesives Age, Nov. 1993, 17.
14. Li, W., Bouzidi, L., Narine, S.S., Ind. Eng. Chem. Res., 2008, 47, 2008.
15. Da Silva, S.A., Marques, C.L., Cardozo, N.S.M., J. Adhesion, 2012, 88, 187.
16. Khaksar, M.R., Khansari, M.G., Toxicol. Mech. Methods, 2009, 19, 257.
17. Test Methods for Pressure-Sensitive Adhesive Tapes, 14th ed., Pressure Sensitive Tape Council (PSTC), Northbrook, Ill., 2004.