I am developing an adhesive for an application that requires heat resistance greater than 80°C. A formulation based on a waterborne polyurethane polymer currently provides the best adhesion to the substrate and is a fit for the application process. What approaches can be used to achieve the required level of elevated temperature performance?
A first approach—and an ideal situation—would be to identify a polyurethane dispersion that has the required property profile as supplied. Polyurethanes with excellent hydrolysis resistance are often based on a polyether polyol backbone. These polymers are often very soft with high elongation and show only moderate levels of heat resistance. Products based on polyester polyols can be either amorphous or crystalline, and film properties can vary widely from low modulus to high tensile strength, depending on the raw materials used in the polymer. The manufacturer of the adhesive raw materials can improve heat resistance by increasing the polymer’s molecular weight or by increasing the “hard block” content of the polymer. This is accomplished by including low-molecular-weight polyols or polyamines in the backbone of the polymer.
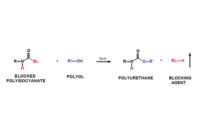
The standard method of increasing an adhesive’s overall properties is through a crosslinking reaction. This increases the polymer’s molecular weight and crosslink density. Polyurethane dispersions are traditionally crosslinked by using water-dispersible polyisocyanates in a two-component process. Isocyanates are available with varying levels of dispersibility. This aqueous mixture is stable for a period of approximately eight hours, and the adhesive should be applied to the substrate during this time period. The bonding process can take place for up to eight hours after the adhesive has been applied. The crosslinking reaction will take place at room temperature over a few days. This type of crosslinking is especially effective for polyurethanes containing reactive hydroxyl groups.
Options are available if a two-component process is not compatible with the bonding operation. One-component crosslinking formulations are available based on both isocyanate and carbodiimide chemistry. One type of one-component crosslinking makes use of an encapsulated solid isocyanate. The isocyanates can be based on the dimer of toluene diisocyanate or the trimer of isophorone diisocyanate. These polyisocyanates are surface deactivated and produce a room-temperature-stable adhesive after being mixed with the polyurethane dispersion. The “wet” adhesive formulations have a pot life of three to six months, while the dried adhesive film coated on the substrate has an open time of up to six months before the bonding operation is initiated. The crosslinking reaction takes place at temperatures of approximately 60-90°C, depending on the formulation.
Carbodiimide crosslinking is possible with polyurethane dispersions stabilized with a carboxylate group. Hydrophilically modified aliphatic carbodiimides are available that can be mixed with the dispersion to produce a one-component adhesive with a pot life of six months. In contrast to the isocyanate crosslinkers described previously, these formulations have an open time of < 1 hour. The short open time indicates these adhesives cure very rapidly in the dried adhesive film. The formulating options available with polyurethane chemistry can be tailored to meet demanding application requirements.