
Rubber-based systems have always been able to perform well on low surface energy materials, but they often exhibit poor stability over time and limited cohesive strength - part of the reason that led to the increased use of acrylic systems. We introduced our developments with rubber-acrylic hybrid adhesives1 at the 2001 PSTC meeting. These new systems were designed to fill a void in the pressure-sensitive adhesive industry. Our hybrid polymer systems combine the best performance attributes of both acrylic and rubber systems in a single material. This article discusses our most recent developments - an increased understanding of how these systems behave and perform, together with improvements to our original material.

The Rubber-Acrylic Hybrid Polymer
To create a polymer with all of the benefits of an acrylic and a rubber, we chose to create a graft copolymer using an acrylic backbone and a saturated hydrocarbon side chain - an approach first described by Mallya and Smith2and shown schematically in Figure 1.
The acrylic backbone contains the typical elements of a pressure-sensitive acrylic: low-Tg monomers (such as 2-ethylhexyl acrylate, butyl acrylate or iso-octyl acrylate) to give tack, higher Tg monomers (such as methyl acrylate or methyl methacrylate) to give cohesive strength, and functional monomers (such as acrylic acid or 2-hydroxyethyl acrylate) to give specific adhesion as well as the possibility of sites for metal crosslinkers to bind. The hydrocarbon side chains are a low-molecular-weight poly(ethylene-butylene) polymer with a methacrylate end group to allow it to be used as a comonomer in the polymerization reaction. The ethylene-butylene polymer has a molecular weight of 4000 and a glass-transition temperature (Tg) of -63 degrees C.
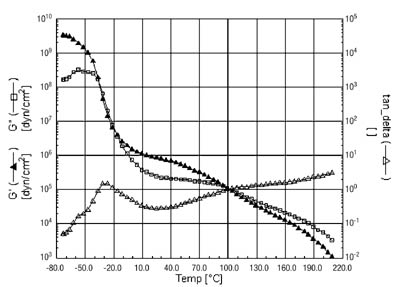
Prior to tackification, the polymer has two distinct Tgs, with the rubber being lower than the acrylic phase. This is shown in the DMA curve in Figure 3.
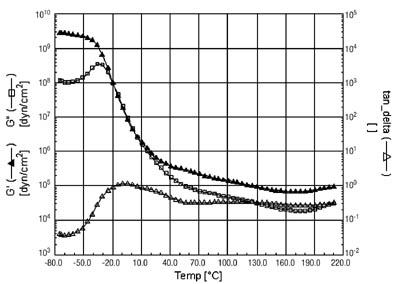
When the polymer is tackified with a hydrocarbon tackifier, the Tg of the acrylic phase remains unchanged. This tackifier is incompatible with the acrylic phase and so does not interact with it. The Tg of the rubber phase is shifted to a higher temperature, now becoming a shoulder on the high temperature side of the acrylic peak. This can be seen in the DMA curve in Figure 4. The adhesive has also been crosslinked, giving rise to a relatively flat storage modulus (G') at elevated temperatures.
Understanding the System
Over the past year, we have spent significant time looking at the performance and behavior of our acrylic hybrid system and attempting to understand some of the causes of the interesting performance we see. Our results are as follows.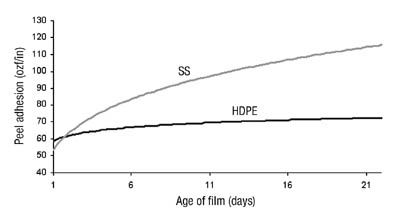
Phase Morphology
When the polymer was tackified with a C5-based hydrocarbon resin, we noticed some interesting changes in performance properties over time. Peel adhesion tests, using a 20-minute dwell time, showed differing results, depending upon the age of the film. A recently coated film would give a lower peel value than a one- to two-week-old film. Through systematic testing, we discovered that the peel value does increase as the film is stored on the liner, reaching a maximum value after approximately two weeks (see Figure 5). As the peel increases, the failure mode begins to change from adhesive to cohesive. Once the peel from steel reaches a maximum value of approximately 120 ozf/in, with a film around two weeks old, the failure is entirely cohesive.
We had hypothesized that this was due to changes in the phase morphology of the system. The two distinct phases would most likely rearrange slowly within the film. The lower energy tackified rubber phase would orient itself toward the low-energy surface of the release liner. Until recently, we had been unable to prove this theory. Several attempts with microscopic techniques, including atomic force microscopy, had failed. This can, in part, be attributed to the fact that the glass-transition temperatures of the acrylic phase and the tackified rubber phase are close together, making distinction difficult. The use of sum frequency generation IR spectroscopy, however, has yielded some interesting results.
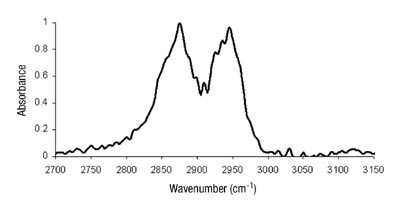
Kinetics of Chelation
We had chosen a titanium-based crosslinker system for our adhesive. In order to provide sufficient stability to the adhesive before coating and curing, a moderately high level of pentane-2,4-dione was added as a stabilizer. While this pentanedione provides excellent storage stability for solventborne adhesives, it presents an additional problem - it can affect the rate of cure. Pentanedione is not particularly volatile and so it evaporates only slowly in the drying oven. Longer residence times and higher temperatures will result in greater removal and therefore greater degree of crosslinking. Obviously, this can start to present economic challenges to the coater and therefore could easily become undesirable. An excellent way to look at the affect of curing conditions on adhesive performance is by measuring the shear adhesion failure temperature (SAFT). An example of this is shown in Figure 8.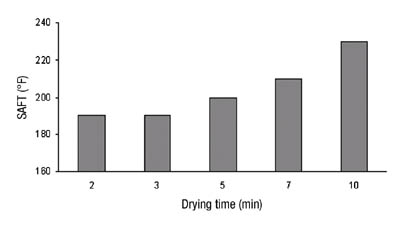
This critical dependence of film performance on curing conditions was one of the motivations that we had to create an improved system that would be easier to use.

New Improvements to the Acrylic Hybrid System
Although we were very pleased with our initial system, improvements could be made. The system performs extremely well in many different environments and is a significant improvement over a rosin ester tackified acrylic for adhesion to low-surface-energy material. The aging characteristics of the material are also better than many simple tackified acrylics and will not give any concern in many applications. Users who subject the adhesive to extremely challenging environments, such as high heat or extended exposure to ultraviolet light, may begin to see problems with stability. The hydrocarbon tackifier we used contains unsaturation, which leads easily to oxidative degradation. In addition, our titanium crosslinking system requires substantial stabilization in the unformulated material. The lack of volatility of the pentane-2,4-dione used for stability has already been discussed. While it is fairly straightforward to achieve sufficient cure to see the desired performance properties, residual pentanedione is not desirable. It will slowly evaporate if the tape is exposed to high-heat environments and therefore result in additional crosslinking and stiffening of the adhesive and a reduction in peel adhesion. If the tape has already been used to form a secure bond before such heat exposure, there will be little noticeable effect. If, however, the tape is exposed to heat before being used to form a bond, the resulting bond strength will be compromised as the stiffer adhesive has lower peel and tack values.
A number of alternative crosslinker systems could have been used. We could have moved away from a transition metal system completely to materials such as isocyanates. These would react with hydroxyl groups in the polymer side chains and give strong, stable covalent crosslinks. Such materials have to be formulated immediately prior to use, resulting in more complex handling for the tape producer. We therefore chose to focus on crosslinking systems that could be pre-formulated into the adhesive and supplied as a one-part system. Isocyanates are still a possibility in such as system. A number of blocked isocyanate materials are available. Such materials have protecting groups to prevent them from crosslinking the adhesive during formulation and storage in solution. The protecting groups are heat labile and therefore are removed in the drying oven and can then crosslink the adhesive film. We found this to be a difficult concept to execute successfully. The temperatures required to activate the blocked isocyanates were generally greater than the temperatures achieved during the normal drying conditions of pressure-sensitive adhesive films. The films, therefore, were normally under-cured and did not exhibit the desired performance characteristics.
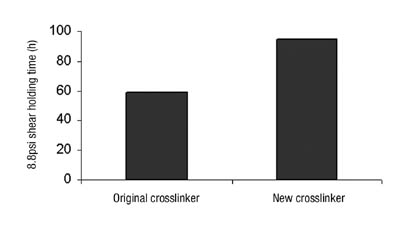
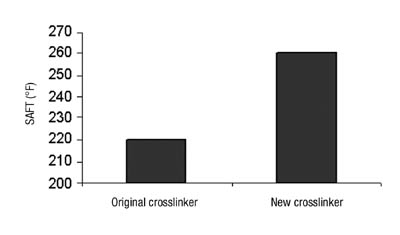
Our shear strength was improved, as was our SAFT performance, which was particularly beneficial (see Figure 10).
Future Developments
Work is continuing on the acrylic hybrid platform and we are investigating a range of opportunities. These include enhanced foam bonding, with particular attention to polyurethane foams, as well as continued work in low-color, environmentally stable hybrid adhesives. We would like to produce an adhesive with a SAFT above 300 degrees F that will still have outstanding adhesion to many substrates and be straightforward to coat and cure.Acknowledgements
I would like to thank the Pressure Sensitive Adhesive Research and Development group at National Starch, but particularly JoAnn Hansen and Brent Sellers, who performed most of the formulation and testing work, as well as Jim Miller for polymer synthesis. Also, Dr. Paul Foreman, Dr. Jennifer Jensen and Dr. Clay Kellam for their technical insight and discussions, and Dr. Rama Chandran for technical advice and management support. Finally, I would like to thank Ali Dhinojwala and Gary Harp at the University of Akron for permission to reproduce their results.This article is based on a paper presented at PSTC's TECH XXVI Technical Seminar, May 2003 in Washington, D.C.
References
1. Foreman, P.; Eaton, P.; Shah, S.Pressure Sensitive Tape Council TECH XXIV, May 2001.
2. Mallya, P.; Smith,
C.C. U. S. Patent 5,625,005; April 29, 1997.
3. For examples of the technique, see:
Harp, G.R.; Gautam, K.S.; Dhinojwala, A.
J. Am. Chem. Soc., 2002, 124, 7908.
Gautam, K.S.; Dhinojwala, A.
Macromolecules, 2001, 34, 1137.
Further references can be found in these papers.


