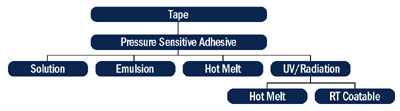
Most of the UV-cured PSAs offered today were nonexistent just a few years ago. Recent years have seen a surge in the number of research publications on UV-curable PSAs, and manufacturers now recognize the benefits of UV-cured PSAs (Table 1). New polymers are being developed, new formulation strategies are being put forth and improvements are being made in the lamp technology.
Overview of the Manufacturing Process
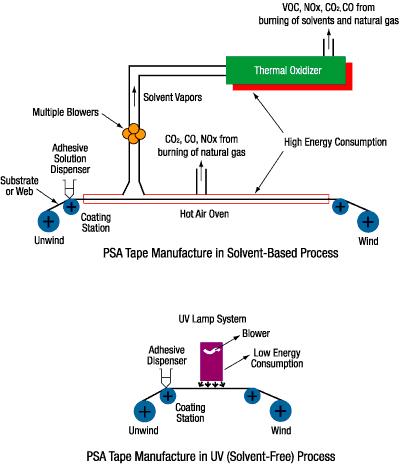
The flow chart in Figure 2 gives a general overview of the manufacturing processes for both solvent-based PSAs and for UV-curable PSA tapes. The flow chart is for illustration purposes; the specifics of each process may differ.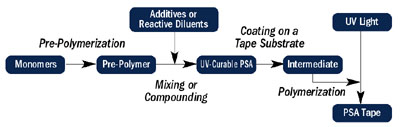
During prepolymer formation or compounding, a radiation-sensitive chemistry is incorporated into the adhesive system. The formulated PSA is then coated on a substrate using a suitable coating technique determined by the viscosity and process temperature, followed by radiation cure.


Curing creates changes at a molecular level. The primary curing reaction in a hot melt UV PSA is the formation of crosslinks (Figure 4), whereas in a RT-coatable PSA, it is chain extension (Figure 5).
The Xs represent the UV-reactive groups that help form crosslinks with other chains. Curing increases the effective chain length (molecular weight) and improves mechanical and thermal properties.
The molecular weight of polymers in RT-coatable UV PSAs is much lower than that of hot melts. Therefore, the former requires a fast and highly efficient curing reaction for the adhesive to attain a respectable molecular weight.
RT-Coatable UV PSAs
RT-coatable UV PSAs are a relatively new concept. These PSAs have viscosities that allow for coating at ambient temperature using conventional roll-coating equipment. The compositional aspects of different RT-coatable UV PSAs are proprietary, but all are solutions of a high-molecular-weight polymer in a UV-reactive diluent.Examples of RT-coatable PSAs (Table 2, pg. 56) may include solutions of a thermoplastic elastomer in acrylic monomer, solutions of acrylated acrylate polymer in acrylic monomer and solutions of acrylated urethane in acrylic monomer. When irradiated with UV in the presence of a photoinitiator, the acrylic monomer reacts to form a polymer that becomes an integral part of the adhesive. The commonly used reactive diluents are 2-ethylhexylacrylate, ethoxylated nonylphenol acrylate and ethoxylated ethylacrylate. Viscosities of RT-coatable UV PSAs are well suited for screen-printing. The most common application is for inline adhesive coating and printing to make nameplates and decals.
In his recent work, Glotfelter1 described the breadth of raw materials exemplified by urethane acrylates and other elastomeric oligomers available for formulating RT-coatable UV PSAs. Quirk2 has reported a UV-curable PSA formulation based on an aromatic urethane acrylate oligomer mixed with tackifiers and acrylic monomers. His publication discusses the formulation details and the use of experimental design to optimize the adhesive properties.
In another work, Dougherty et al3 described the use of vinyl ethers in conjunction with cationic photoinitiators to form a PSA. They copolymerize a low-Tg and high-Tg vinyl ether in the presence of varying amounts of tackifying resin. Decent PSA properties have been reported.
A recently patented strategy is based on forming acrylic syrup.4 Acrylic monomers are partially polymerized to a syrup consistency of low-molecular-weight acrylated copolymers called “A” stage. The syrup is usually coated on the web and exposed to UV radiation to complete the polymerization reaction. A typical formulation may be composed of 50% to 99% of monofunctional, and 50% to 1% of higher functional monomers. The Tg desired from the fully cured polymer dictates the choice of monomers. This approach provides a high degree of freedom in the design of polymer structure but is less common because difficulties controlling the consistency of the syrup result in reproducibility problems.
UV-curing technology is also impacting conventional PSAs. For example, Fukui et al5 have described a novel PSA strategy blending a cationically curable epoxy in a PSA formulation. This adhesive behaves as a conventional PSA, but after UV curing, its bond strength builds to a structural-type adhesive.
Great strides have been taken towards developing a RT-coatable UV PSA but any new development comes with its share of challenges (Table 3). As the technology grows, however, these concerns will likely be resolved.
Hot Melt/Warm Melt PSAs
Hot melt/warm melt PSAs have a more sophisticated design technology that circumvents some of the issues surrounding RT-coatable UV PSAs. The available hot melt/warm melt PSAs are classified by the type of base polymer used in the adhesive (Table 4). The base polymers have a relatively high molecular weight that melts under heat.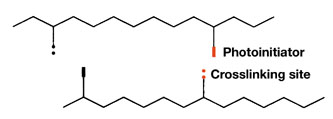
Acrylic Warm Melt PSAs
The development and the various opportunities of UV-curable polyacrylate warm melt PSAs are discussed by Barwich et al.6The polymer chains of this acrylic warm melt PSA are carefully controlled in molecular weight and distribution to flow at a relatively sharp and low temperature. Their viscosities drop rapidly on heating to enable processing at elevated temperature.A polyacrylate, which is at the upper viscosity limit when processed as a hot melt, does not have a sufficiently high molecular weight for satisfactory PSA application. The polymer molecules must either have a very high molecular weight or be crosslinked by additional chemical bonds to exhibit good adhesive properties.
A typical polyacrylate is made by solution polymerization, and the solvent is stripped after the process. A properly formulated copolymer is capable of being crosslinked in the presence of photoinitiator and UV radiation. A prevalent crosslinking strategy is to incorporate the photoinitiator units in the backbone of the copolymer to provide crosslinkable sites (Figure 6, pg. 58).
Good adhesive properties are achieved when the crosslink density is low because the molecules can still flow and therefore cause rapid wetting of the surface to be bonded. The efficiency of the crosslinking reaction is low; therefore, an excess of photoinitiator and crosslinking sites are incorporated into the copolymer.
As the polymer is irradiated with UV, crosslinking achieves a good balance of peel and shear but extra crosslinking sites remain. The polymer is capable of forming more crosslinks with increased UV dose, thereby enabling tailored properties to be derived from the system. However, it does not take too much to under-cure or over-cure these adhesive systems, which hurts the balance of properties.
Acrylic copolymers are inherently pressure sensitive and normally would not require any additional tackification. Innovative blending of resins through heating and mixing in their molten states can modify the properties of these adhesives.
The processing temperature for these adhesive systems ranges from 120°C to 140°C. They are typically applied using a slot-die method.

Block Copolymers in Hot Melt/Warm Melt PSAs
Two block polymers have recently been developed, one to create a warm melt and the other a hot melt UV PSA. The warm melt starts with a base diblock polymer made by sequential polymerization of isoprene and butadiene monomers that is hydrogenated and functionalized with multiple epoxy groups on one end and a hydroxyl on the other to make it reactive (Figure 7). Tight control of molecular weight yields a highly viscous polymer at room temperature that fluidizes at elevated temperature. This system is usually processed at 120°C and coated on the web using a slot die.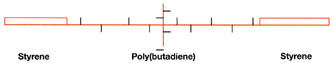
This system attains good pressure sensitive properties only in the presence of tackifiers. The ratio of the epoxy polymer to the hydroxyl-terminated polymer and the amount and the type of tackifier can be varied to tailor the adhesive properties. Increasing the fraction of the hydroxyl-terminated polymer will reduce the crosslink density. The maximum achievable crosslink density is determined by the epoxy-to-hydroxyl ratio, thus preventing over-curing.
Adhesive thickness and UV dose determine what percentage of maximum achievable cure is actually obtained. The photoinitiator package must also be optimized for a given adhesive thickness and UV dose. To obtain a consistent product in a fail-safe manner, one should customize the formulation so that the maximum achievable cure is reached.
Erickson and Mancinelli7 have performed a very detailed study on this diblock polymer system for use in high-performance PSA applications. They have discussed the formulation and curing techniques, and report the relevant test results.
Another type of block-polymer system available is based on styrene-butadiene-styrene triblock polymer. As shown in Figure 8, this polymer is a rubbery solid at room temperature with a relatively high molecular weight that requires a temperature of about 160ºC to melt and process the polymer.
This polymer is designed so that the center polybutadiene block bears a high level of pendant vinyl groups. The vinyl groups on this polymer are capable of reacting in the presence of free-radical photoinitiators and UV radiation to form crosslinks. A high concentration of vinyl groups is needed to compensate for the inefficient crosslinking reaction. Crosslink density builds as the UV dose increases, achieving an optimum balance of PSA properties. At this point, the polymer still has unreacted vinyl groups, but increasing the UV dose deteriorates PSA properties.
This polymer requires the use of tackifiers to exhibit pressure sensitive properties. The properties depend on the type and amount of tackifier, the UV dose and the thickness of the adhesive. It is very easy to under-cure or over-cure an adhesive based on the triblock polymer by slight variations in thickness and UV dose.
Smith8 recently discussed the use of the triblock polymer to formulate a UV hot melt PSA. He reports data to demonstrate the improvement in high-temperature properties obtained in going from an uncured to a cured state.
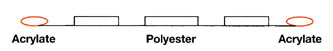
Polyester Warm Melt PSAs
A series of PSAs are based on polyester as the main phase – amorphous copolyesters with acrylic end groups on either side. As illustrated in Figure 9, the polymer is saturated in the main chain and has a Tg ranging from -50°C to -10°C.The presence of terminal acrylic end groups renders them radiation curable in the presence of photoinitiator. Acrylic functionalization is less than 100% since moderate crosslinking is needed to attain pressure sensitive properties. Designed as single-component adhesives with little or no reactive diluents or tackifier, they must be heated to 100°C to 140°C to achieve a coatable melt viscosity. A slot-die coating method is usually suitable. Polyester warm melt adhesives find applications in automotive labels, tapes, transfer tapes and UV-resistant tapes.
In another development reported by Kauffman et al9, acrylated polyesters have been used in warm melt as well as RT-coatable UV PSAs. The polyester in its neat state acts as a warm melt UV PSA. The same polyester after being formulated with tackifying resins and acrylic monomer functions as a RT-coatable UV PSA. Decent peel and shear values have been reported.
Formulation Aspects of PSAs
The logistics of design for UV PSAs are similar to those of solution PSAs. Three stages have been identified in Figure 10 where the properties of the PSA tape can be controlled. The first stage is the identity and composition of the monomer raw material, which will affect the inherent polymer. The architecture of the polymer, its molecular weight and distribution, and the functionality have a strong bearing on the properties of the final tape.The second stage is mixing, where control of properties is achieved by selection of tackifiers, fillers and the crosslinking agents. Potential options include adding high- or low-melting tackifiers to alter the shear and peel strength, adding carbon and metal particles to form an electrically conductive adhesive, enhancing temperature resistance and shear property with crosslinkers, and adding antioxidants to enhance the aging properties.
In the last stage of curing, UV dose determines the extent of the reaction and the crosslink density. In contrast to a solvent PSA, UV PSAs offer an extra degree of freedom to develop tailor-made adhesive properties. A slight variation in dose and intensity can, however, also lead to an inconsistent product.

Coating and Curing PSAs
The time and money required to convert a formulation to a tape must not be underestimated because formulations undergo a chemical reaction on the substrate after being coated. It is also essential to obtain reproducible properties with the curing process used. It is important that we put down a uniform thickness, ensure that the coating is inerted, ensure that the composition is preserved as the formulation is cured, ensure that the formulation attains the expected degree of cure and obtain the tape construction desired by the customer.Like solvent-based formulations, UV formulations are fluid and conform to the same laws of physics and fluid mechanics. Viscosity, viscosity under shear, surface tension, process speed and other factors play a critical role in the quality of coating. The wide range of viscosities resulting from the various formulation strategies makes it important to experiment with the configuration and the type of coating head. The lamp configuration and the photoinitiator levels must be experimented with to obtain the optimum lamp power and to achieve just the right amount of cure.
The effect of UV dose on adhesion and cohesion is shown in Figure 11, emphasizing the necessity of cure optimization. It shows that as the UV dose increases, the tack decreases and the cohesion increases. Peel adhesion and high-temperature shear (SAFT) are also shown to depend on the degree of cure. Both curves pass through the “cure window,” a plateau representing optimum conditions for curing. The exact position of the curing window must be determined for every formulation and each thickness.
Challenges
UV-lamp technology, raw-materials availability and innovative formulation strategies have seen a tremendous growth in recent years, taking PSA technology to a new level. It is a living, continuously improving technology, but some challenges are still present (Table 5). However, some education on the part of formulators and converters is needed to translate the technology to a product.An understanding of the chemistry and physics of the adhesive platform is essential for a successful tape product. The well-known law of physics that the intensity of light falls exponentially as it travels through an absorbing medium means the cured adhesive can develop a crosslink density gradient through the coating. This gradient may manifest in providing distinct properties on the top and bottom surfaces of the adhesive. By applying the principles of radiation curing, this gradient can be minimized or exaggerated. For instance, a system that has been optimized for a two-mil-thick adhesive will not reach optimum cure at five mils of coating requiring tighter process controls.
The UV process window for PSAs is narrow, and the adhesive properties are a function of radiation dose, making it is easy to either under- or over-cure the adhesive. A good understanding of the relationships between radiation dose and radiation intensity on cure chemistry is required. Also, adhesive formulators need to design adhesives that can tolerate variations in curing conditions without compromising performance. Moreover, developments in feedback loop controls are beginning to take this variable out of the equation.
Care should be taken to design curing systems that circumvent the effects of oxygen inhibition on free-radical reactions. Even the slightest inhibition can throw a tape out of its narrow process window. Improvements in photoinitiator packages are being made that keep stretching the scope of the technology. The result is a faster reaction, lower loading of the photoinitiator and reduced out-gassing of residuals. The introduction of warm/hot melt UV PSAs has overcome the undesirable monomer odor found in a majority of RT-coatable PSAs.
An essential requirement of UV curing is that the adhesive has to be transparent to the UV light in order to be cured. Filled or pigmented adhesives may pose a curing challenge. However, the E-beam technology, similar in principal to UV, can fill in this void.
The issues discussed above may sound limiting, but the challenges can be met by combining the emerging technology with proper education of the variables and how they interact with each other, and the innovation of a savvy operator. This technology provides unique benefits that cannot be achieved by existing technologies.
Conclusion
The developments in various formulation strategies and the types of UV-curable PSAs available have been discussed. It has also been recognized that UV PSA technology is still in its infancy, and, therefore, a lot remains to be discovered. The current library of raw materials is limited, as is the experience in an actual production setup. Because the properties of the adhesive can be adjusted right up to the curing stage, there is currently no such thing as a standard radiation-curable PSA. The chemistry must be adjusted to the process or vice versa. Because chemical reaction takes place during curing, a good understanding of the process and chemistry and how these two interact is needed to make a consistent product. A hundred-percent conversion should be achieved in order to reduce residual monomers in the tape and to achieve a consistent product. However, the recent developments in the warm melt block polymer and the warm melt acrylate polymers have not only solved the problem of residual monomer but also provide a distinct performance advantage over conventional hot melt PSAs.Additional information on radiation-curable pressure sensitive adhesives is available from Adhesives Research, Inc., 400 Seaks Run Road, Glen Rock, PA 17327; phone 800-445-6240, ext. 3294 (717-235-7979 outside the United States); fax 717-227-0973; e-mail rmalik@arglobal.com.
