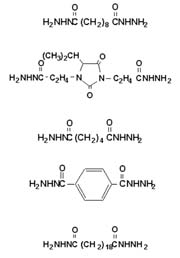
Although hydrazine is a suspected carcinogen, the safety of ADH has been well established.2 IDH is approved by the FDA for indirect food contact when used to cure epoxy resins under 21 CFR 175.300.
Epoxy Resins
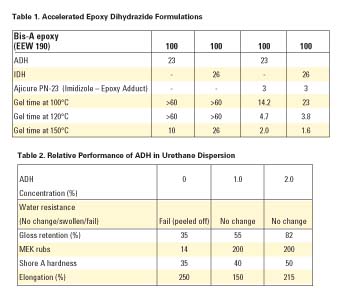
In epoxy resins, dihydrazides are typically formulated to 1/4 of dihydrazide to each epoxy equivalent. That is, all four of the primary hydrogens will react, each with one epoxy group. In our formulations, we have found that 0.7-1.2 equivalents of active hydrogen per equivalent weight of epoxide exhibit acceptable cures with little to no reduction in properties. The epoxy-cure temperature is related to the melt temperature of the dihydrazide. However, the dihydrazides can be accelerated with various free-electron-donating compounds, such as ureas, imidazoles and imidazole adducts, as well as inorganic compounds like lead octoate and stannous octoate, with some effect on pot life.Dihydrazide exhibits a lower onset cure temperature than dicyandiamide (DICY), but can be accelerated in the same manner. Dihydrazides can be accelerated in same manner as dicyandiamide. We experimented with ADH and IDH (see Table 1), however the same principals hold true to all dihydrazides.
An advantage of the dihydrazide epoxy combination is that it is B-stageable, allowing for use in prepregs, adhesive films, molded parts, etc. Prepregs made of ADH and IDH can be B-staged at temperatures up to 165°C and are storage-stable for up to three months at room temperature. Cured prepregs with ADH will show a weight gain of about 2% after 1 week in boiling water. Prepregs cured with IDH exhibit weight gains of less than 1% under the same conditions. Lap shears at elevated temperature show little to no strength loss, and epoxies cured with dihydrazide show unparalleled toughness. Dihydrazide epoxy compounds can be formulated as flexible systems. 3
Urethanes
Dihydrazides will cure an isocyanate through the primary amine to form the urea. This reaction is useful as a chain extender and crosslinking agent for urethane adhesives, coatings, and emulsions. ADH is water soluble.We blended ADH with a mixture of an isophorone-diisocyanate-based urethane emulsion with a hexamethylene-diisocyanate emulsion. We then added 0-2% ADH, based on the total emulsion. Solvent-degreased steel plates were painted with a 6-mil-thick coating and dried overnight at ambient temperature and humidity. The plates were then submersed in water for 24 hours or submitted to a Weatherometer test for 1,500 hours, and 60° gloss retention was measured. Additionally, 1/8"-thick sections of the urethane film were measured for durometer hardness as well as elongation. We only wanted to find the relative performance of the ADH, not to actually optimize the performance of the coating. The results of these tests are shown in Table 2.
Dabi4 et al. describes that dihydrazides improve the thermal oxidation color stability of polyurethanes. They made urethane emulsions of dimethyol propionic acid and hexamethylene diisocyanate, or toluene diisocyanate, and chain-extended these emulsions with ethylene diamine. Cast films based on these polymers yellowed at 185°C. The addition of ADH or CDH in a concentration as low as 0.25% completely eliminated the yellowing. Dabi also notes that the urethanes that are chain-extended with a combination of a diamine and a dihydrazide yield superior tensile strength over either of these individual amines.
Along the same lines, Hirai5 et al describes the use of IDH for manufacturing leather-like coatings of solution-based polyurethanes. A combination of isophorone diamine and IDH used as a chain extender for a diphenylmethane diisocyanate (MDI) and polycaprolactone diol yields a urethane with Tgs
of -40°C with excellent thermal stability,
as well resistance to hydrolysis and
solution stability.

R1 is the Acrylic Backbone
R2 is the Remainder of the Dihydrazide
Acrylics
Dihydrazides will cure acrylics by Michael's Addition, which is a very quick reaction. Much work has been done on using water-soluble dihydrazides, such as ADH and VDH. In addition, the dihydrazides will react with the free aldehyde of the acrolein in most acrylic coating to form a pendant hydrazone. This hydrazone continues to react with other free acrolein to crosslink the acrylic polymer, as shown in Figure 2.
Dihydrazide-containing polymers show improved wet-rub resistance and better Mandrel-bend resistance, as well as improved Weatherometer resistance.
Summary
Dihydrazides offer versatility for the formulator in preparing one-part epoxy systems. They have a lower onset temperature than DICY, are fully reactive and have excellent B-stage properties, toughness, and adhesion. In urethane emulsions and solution urethanes, the dihydrazides offer additional crosslink density and chain extension, and they also reduce yellowing, which is counterintuitive to what amines normally do in urethanes. Dihydrazides offer a high measure of crosslink density for solution-based acrylic coatings.Acknowledgements
We would like to thank Ajinomoto-Fine-Techno for supplying us with much of the epoxy data, as well as the dihydrazides we have used.
For more information on curing agents, contact A&C Catalysts, 333 Hamilton Blvd., S. Plainfield, NJ 07080; phone (908) 226-7575; fax (908) 226-7577; e-mail sales@ac-catalysts.com; or visit http://www.ac-catalysts.com.
References
1 US Patent #2847395. "Stable Heat-Curing Epoxy Resin Compositions." Wear.2 "Final Report on the Safety Assessment of Adipic Acid Dihydrazide," Journal of American Coll. Toxicology (1994) 13(3) 154-156.
3 US Patent #5965673. "Epoxy-Terminated Prepolymer of Polyepoxide and Diamine with Curing Agent." Hermansen et al.
4 US Patent #4447571. "Stabilization of Polyurethanes." Dabi et al.
5 US Patent #4412022. "Polyurethane Compositions Prepared from a Polymeric Diol." Hirai et al.
Lee and Neville, Handbook of Epoxy Resins. Pp. 10-18.