The DSC technique is not new, but its utility for many kinds of analysis has recently been greatly enhanced by TA Instruments' introduction of the Q Series line of DSCs incorporating TzeroT Technology (Figure 1). This development is not just another DSC cell with improved baseline stability, but in fact a new measurement principle with an additional sensor that together provide compensation for intrinsic instrument distortion of the DSC signal2. Because distortion has been virtually eliminated, it is now possible to measure specific-heat capacity directly in a single scan, and to achieve greater sensitivity and accuracy. Tzero Technology also reduces analysis time, especially for Modulated Temperature Differential Scanning Calorimetry (MDSCR)3.

Glass-Transition and Viscoelastic Properties
Most adhesives take advantage of the viscoelastic behavior of polymers. The long chains of macromolecules in the amorphous phase exhibit a wide range of properties, from viscous flow to rigid solid, depending on the temperature. The properties change most rapidly, but continuously, through the Tg region. For example, the viscosity typically changes by up to several orders of magnitude over this region. So, at the high-temperature end of the Tg region, the molecules can easily flow into the interstices of the surfaces to be bonded, while at the low-temperature end of a Tg region, the material achieves adequate mechanical strength. Thus, the design of an effective adhesive is one whose Tg allows sufficient flow at the application temperature and sufficient strength at the service temperature.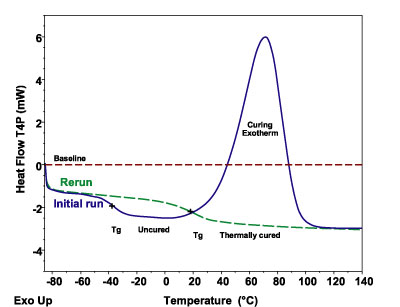
Epoxy Curing
To see how this is accomplished in a familiar material, consider the curing of a household two-part epoxy in the DSC. Figure 2 shows three curves: the first, labeled "Initial Run," is the epoxy sample as it is heated from low temperature at a constant heating rate. As the mixed epoxy is heated through the Tg region, the molecules gain sufficient mobility to resume the crosslinking reaction, which appears as an exothermic peak. The Tg in this partially cured material is still below room temperature, which indicates that the polymer can flow into the surfaces to be bonded.
In the second curve, labeled "Rerun," a reheat of the sample, the crosslinking has shifted the Tg to a higher temperature where the material has mechanical strength at room temperature. Since the exothermic energy is a direct measure of the bonds that have been formed, measuring the peak area (energy units) can be used to assess crosslink density. For a partially cured system, the residual energy can indicate degree of cure, as can the extent of shift in the Tg between the uncured and fully cured states.

Pressure Sensitive Adhesives
In a pressure sensitive adhesive (PSA), there is no mechanism to shift Tg after bonding as there is in a cured, or solvent-dried, system. For many applications, the combination of bonding tack, peel strength and shear resistance must all be achieved simultaneously for the full range of application and use. To accomplish this, the formulation provides a mix of viscoelastic components characterized by broad or multiple transitions.
Figure 3 shows the DSC analysis of three PSA formulations. Interpretation is complex since each sample appears to have a glass transition and one or more first-order peaks. From the change in heat capacity (DCp) at the Tg, we can obtain a measure of the amount of material in each amorphous phase. The area of endothermic peaks gives the amount of material in any crystalline phase. While Figure 3 shows differences between the formulations, the fact that DSC measures only the sum of all the thermal events can complicate the analysis when overlapping processes occur at the same temperature. In fact, it is difficult to unambiguously pick the beginning and end of the transitions from this data. Fortunately, Modulated DSCR (see MDSC Aids Interpretation on pg. 40) is available to deliver additional information by effecting a separation of the processes involved.
Improved Technology
While the DSC technique is straightforward for analyzing simple systems, its use for quantifying complex mixtures requires more from the instrumentation and methodology.
Baselines. When transitions extend over tens of degrees it is especially important to have a straight instrumental baseline. If this cannot be achieved, then the same curvature that appears in the instrument baseline is superimposed on the sample data. Then, when the constructs are placed to determine the glass transition, the curvature will result in an incorrect placement of the Tg and compromised determination of DCp at Tg. This problem is especially troublesome when high sensitivity is required to observe a subtle transition.
Tzero Technology improves baseline straightness by an order of magnitude over previous DSC instrumentation. Figure 2 shows a typical instrument baseline for an adhesives analysis. Notice that the baseline is not only flat but also is at zero milliwatts. This is because Tzero Technology effectively removes the causes of instrumental baseline offset and curvature.

Specific-Heat Capacity
This is the fundamental thermodynamic property needed to analyze materials. The normal output of a DSC is heat flow, which includes the specific heat of the sample (times the sample weight and scan rate) plus the baseline characteristics of the analyzer. Once the signature of the instrument has been removed by using the full four-term heat-flow equation made accessible by Advanced Tzero Technology, the displacement from the zero line (whether heat-flow or specific-heat units) is directly the heat flow to or from the sample specimen. As a result, any DSC analysis can be a specific-heat analysis. It is merely a choice of units.
On other DSCs, the heat-flow signal includes offset, slope and curvature from the instrument, so it is necessary to perform multiple DSC scans with and without a sample, and a third scan using a standard material, in order to determine specific heat.
MDSC Aids Interpretation
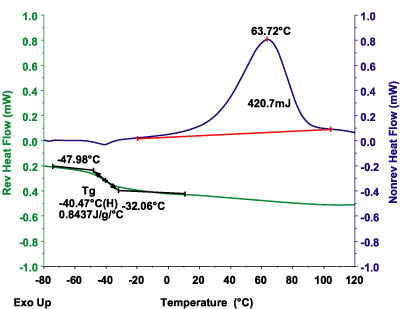
The DSC analysis of multiple-phase polymeric systems is often made complex by the various phase and crystallite equilibria, which shift with temperature. The result can be a complex thermal curve that may contain superimposed glass transitions, crystallization peaks and melting peaks.Modulated DSC is an advanced DSC technology that permits separation of the total heat-flow signal into its more easily interpreted heat-capacity and kinetic-related components3. Figure 4 demonstrates this capability for the above epoxy adhesive, where the glass transition (heat-capacity effect) is cleanly separated from the curing phenomena (kinetic effects). In this way, any volatiles loss at the Tg (kinetic effect) does not adversely effect the Tg determination. Also, the change in Cp, which attends the curing, does not compromise the calculation of the heat of curing, or of kinetic analysis of the curing process.
MDSC greatly aids in the interpretation of the PSA data presented in Figure 3. Each adhesive sample appears to have one glass transition and one or more first-order peaks. However, a more detailed analysis of this material using MDSC shows (Figure 5) that there are two glass transitions in each of these materials, and these are the major determinants of the viscoelastic properties. In fact, once irreversible kinetic effects, such as volatiles release, have been removed, the glass transitions can be obtained accurately from the Cp-related reversing signal.
MDSC is indeed a valuable tool in the interpretation of complex amorphous systems. One previous constraint on its use, namely the slow heating rates required, has been resolved by the new Advanced Tzero Technology. The PSA MDSC data shown in Figure 5 were taken using a temperature-modulation period of just 20 seconds and an underlying heating rate of 10degC/min. This superior quality data was obtained by MDSC in the same time period required for the standard DSC data shown in Figure 3.
Conclusions
DSC has long been used to develop successful adhesive products. Now the Q Series DSC with Advanced Tzero Technology provides a new and enhanced tool for improved design and characterization of adhesives. It offers superior heat-flow accuracy and precision, straighter baselines, better sensitivity and resolution, direct collection of Cp data, and fast MDSC analysis.For additional information on the Q Series line of DSCs incorporating TzeroTM Technology, contact TA Instruments, Inc., 109 Lukens Dr., New Castle, DE 19720; phone 302-427-4000; fax 302-427-4001; visit the Web site www.tainst.com; or e-mail info@tainst.com. Or Circle No. 69.
References
1. S. R. Aubuchon, "Use of Controlled-Stress Rheology to Qualify and Predict Adhesive Performance", Adhesives & Sealants Industry, August 2001, p 46-48.2. R. L. Danley and P. A. Caulfield, "DSC Baseline Improvements Obtained by a Heat Flow Measurement Technique", Proc. 29th Conf. N. Amer. Therm. Anal. Soc. (2001).
3. E. Verdonck, K. Schaap, and L. C. Thomas, International Journal of Pharmaceutics, 1999, 192, 3-20.
