
Several factors drive the demand for silicones in aircraft applications, including materials that perform multiple functions, silicone processing and formulation improvements, and the increased sophistication of manufacturers. Multi-functional material demands - such as ice-phobic, radar-absorbing or static-dissipation, wide operating temperatures, as well as fuel resistance - are increasingly common in this industry. An advancement of silicone technology significant to the aircraft industry is the development of fast-cure, room-temperature-vulcanized (RTV) silicone adhesives with fuel-resistant properties. These materials are extremely versatile and can be designed and formulated to permanently or temporarily bond surfaces together while maintaining excellent elastomeric qualities.
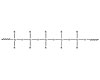
Figure 1. General Structure of Polysilioxane
Silicone Adhesive Chemistry
Silicone adhesives are made up of low-consistency elastomers containing low-viscosity polymers, reinforcing fillers and adhesion promoters. Each component can be modified for a specific process or application. To understand how silicones can be formulated to change basic characteristics, such as adhesive strength or fuel resistance, a brief discussion of silicone polymer and material chemistry is useful.Silicone polymers are inorganic polymers composed of repeating R2Si-O units that have no carbon atoms in the backbone and are called polysiloxane polymers. The structure of silicone accounts for its high degree of flexibility.1Figure 1 shows their typical structure.
The silioxane backbone can be formulated with different types of constituent (R) groups integrated onto the polymer backbone. Typical constituent groups include dimethyl, methylphenyl, diphenyl and trifluoropropylmethyl functionality.
Dimethyl silicones, or dimethylpolysiloxanes, are the most common industrial silicone polymers. They are typically the most cost-effective to produce and generally yield good physical properties. The strength of the Si-O bond gives silicones the inherent ability to withstand extreme temperatures1and environmental conditions while retaining mechanical properties where other adhesive materials may become brittle.
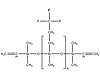
Figure 2. General Structure of a Vinyl Terminatate Trifluoropropylmethyl Polysilioxane Copolymer
Fluorosilicones are based on trifluoropropylmethyl polysiloxane polymers. Figure 2 shows a typical structure for a fluorosilicone copolymer. The trifluoropropyl group contributes a slight polarity to the polymer, resulting in swell resistance to gasoline and jet fuels. Adjusting the amount of trifluoropropylmethyl siloxane units in the polymerization phase provides optimal performance in specific applications. While some fluorosilicones contain 100% trifluoropropylmethylpolysiloxane repeating units, other systems contain a combination of the fluorosiloxane units and dimethyl units to form a copolymer.
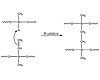
Figure 3. General Platinum Addition Cure Mechanism
The Art of Curing
The curing mechanism is vital to the adhesion of the silicone adhesive to a substrate. The cure chemistry gives the silicone adhesive its cohesive strength. Generally, there are two major categories of silicone adhesives: two-part platinum-addition-curing silicones and one-part condensation cure. Each system requires a functional polymer, crosslinker and a catalyst to cure. The ratio between the components must be balanced to give optimal properties.2Two-part addition-cure systems typically use heat to accelerate cure and often require a primer to the substrate prior to application. Platinum-catalyzed silicones use a platinum complex to participate in a reaction between a hydride functional siloxane polymer and a vinyl-functional siloxane polymer.3The result is an ethyl bridge between the two polymers. The reaction mechanism is illustrated in Figure 3.

Figure 4. Tin Condensation Reaction
Addition-cure systems are subjective to cure inhibition. This is typically observed as either temporary or permanent prevention of a system from curing. Effectively, the crosslink density is disturbed, resulting in weaker material properties, uncatalyzed regions or inconsistency in cure over time. Several types of materials may have ingredients that could potentially inhibit cure; tin, sulfur and some amine-containing compounds may permanently inhibit cure.4Compounds that inhibit cure can be identified easily by attempting to cure a platinum-catalyzed system in contact with the compound.
RTV silicone adhesives generally use an acetoxy or oxime tin-condensation cure system. The advantages of these types of cure systems are their ability to cure at room temperature, robust nature (i.e., difficult to inhibit) and primerless application. This is useful for temperature-sensitive applications.
Condensation-cure systems require atmospheric moisture to cure and release acetic acid as a byproduct. The release of acetic acid results in a vinegar-like smell that fades as evaporation takes place. In some instances, the acetic acid byproduct may help increase adhesion by lightly etching the substrate surface, but must be carefully considered with corrosion-sensitive substrates. A more detailed mechanism of the tin condensation cure mechanism is shown in Figure 4. The figure demonstrates that this type of condensation system involves hydroxyl-functional polymers, alkoxy-functional crosslinking compounds and a tin catalyst. The acetoxy-functional crosslinker first undergoes a hydrolysis step and is left with a hydroxyl group. This hydroxyl group then participates in a condensation reaction with another hydroxyl group attached to the polymer.3The reaction can proceed without the assistance of the tin catalyst, but the presence of the catalyst increases the rate of reaction.
Customers are requesting increased cure time of silicone adhesives for a variety of reasons. For instance, field repair often requires products that cure rapidly. The reduction or removal of process bottlenecks is another driver of demand for fast-cure RTV adhesives. Process engineers often request that an adhesive cure in less than 10 minutes: partial cure (tack free) for handling under five minutes and full cure at room temperature in eight hours, or one shift. Currently, most fast-curing RTV silicone adhesives are specified to cure within 72-77 hours at ambient conditions, with a tack-free time of 15-20 minutes.5However, under optimal conditions (i.e., the right humidity, temperature, and thickness), cure can be drastically increased.

Adhesion and Bonding
Fast-cure RTV silicones with fuel-resistant properties bond aggressively to most substrates. They are designed to adhere to various substrate surfaces, including metals, glass and certain plastics. Chemical reactivity and substrate surface energy are the two critical factors that influence a good surface bond. Chemical adhesion (the chemical bonding of two substrates, by covalent bonding, hydrogen bonding or other Van der Waals forces) is the most influential mechanism to achieve good bonding. Substrates with reactive groups available for bonding - like OH groups on glass and oxide layers on metals like aluminum - make this chemical bond easier to achieve. Substrates with inert surfaces, such as graphite and Polytetrafluoroethylene (PTFE), can make adhesion difficult.6Adequate surface wetting is another critical factor in how the adhesive forms a mechanical or chemical bond to the substrate surface. Surface energy (a determining factor in how an adhesive spreads across a substrate surface) is the major driving force for this phenomenon. It is commonly accepted that the substrate’s surface energy must exceed that of the adhesive for adequate contact.6,7That is, the better an adhesive spreads, the more intimate the contact between molecules. This allows more reactive groups to interact or bond. Therefore, the interactions between the adhesive and substrate make a stronger bond. Polysilioxanes have very low surface energy; a typical surface energy for a silicone elastomer is 24 dynes/cm.7The low surface energy of polysiloxanes is influenced by the substituent R-groups, as previously discussed. This is the reason why silicones are often used as mold-release agents, waterproof coatings and in myriad biomedical applications.8
Surface Preparation Tips
Surface cleanliness is essential for good adhesion. Ensuring that the substrate is free of contamination, such as fingerprints, cutting oils, dust, plating bath residues and other contaminants, is vital. Condensation of certain adhesives’ outgassing products can also contaminate substrates, even if the adhesives are not in contact with the substrate. If using a solvent to clean the surface before dispensing the adhesive, a final rinse with a reagent-grade isopropanol is highly recommended. Plasma surface cleaning may also be effective. In the end, baking the surface first to remove contamination may be the best alternative if contamination, such as epoxy adhesive-derived surface contamination, is not easily removed by cleaning.Conclusion
The evolving needs of the aircraft industry are driving the demand for new technologies and materials. Improvements and advancements in silicone-based materials, such as accelerated-cure RTV fluorosilicones, are accelerating the adoption in new applications. For aircraft adhesive and sealant applications, it is critical to understand the material chemistry and composition options for optimal performance. Further understanding of bonding mechanisms will aid in improving material selection and processing techniques.For more information, visit www.nusil.com.

