
Figure 1: Chemical formula of an aromatic polyisocyanate
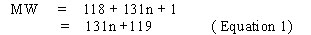


For phenyl isocyanate, where n=0, it follows that MW = 119, IE= 119, %NCO = 35.29 In the case of MDI, where n=1, the calculations lead to the following values MW = 250, IE = 125, %NCO = 33.60

IE and %NCO of polymerics
Whenever the polyisocyanates are the result of a polymerisation process, n assumes an average value. The equivalent weight of species with an n value approaching infinity can be calculated as follows.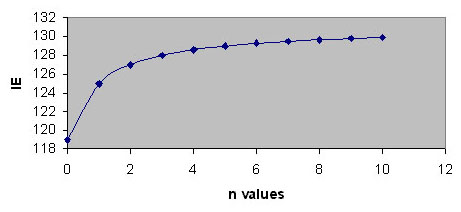
Figure 2: Variation of isocyanate equivalent with the number of repeat units
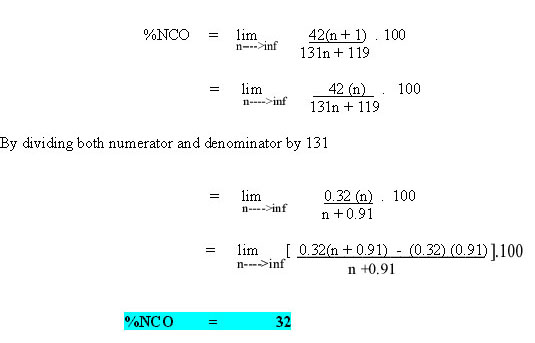

Figure 3: Variation of NCO content with the number of repeat units
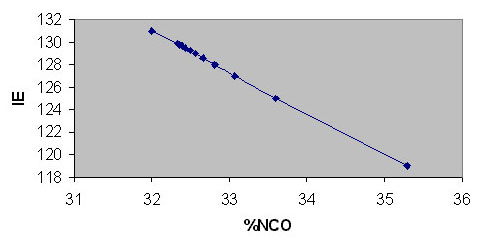
Figure 4: %NCO for different IE values
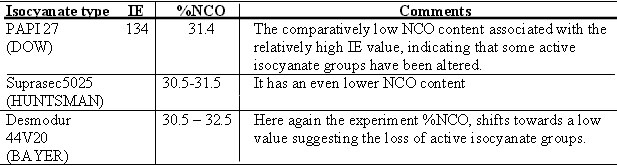
Table