What distinguishes hot melts from other adhesive types is that they are applied in their molten state (usually 175-205 degrees C), and then rapidly cooled to form a tough, adherent solid at room temperature. Their rapid set time, coupled with their relatively high viscosity, makes them ideal for bonding porous materials. Hot melts find numerous applications in a variety of manufacturing processes, including box and carton sealing, bookbinding, footwear, and labeling.
What is a Hot Melt?
Hot-melt adhesives are generally thermoplastic polymers of relatively high molecular weight (MW), giving them both strength and high viscosity. But high MW polymers alone generally do not have sufficient adhesion (tack), so these polymers are blended with a variety of additives, which can include plasticizers, tackifiers and stabilizers, to increase adhesive performance. Since hot melts are classified as thermoplastics, exposure to high temperatures can cause the adhesive to re-melt. Therefore, most hot-melt applications are limited to room temperature and near room temperature applications.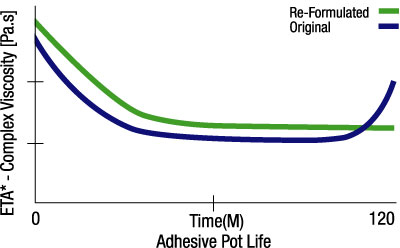
Why Rheology is Important
Rheology can be defined as the study of the deformation and flow of materials. Typically, materials exhibit both elastic (the ability to store energy) and viscous (the ability to dissipate energy in the form of heat) behavior. These behaviors change as a function of temperature, time and rate or degree of deformation.Hot-melt adhesives are applied in a molten state, and most must flow smoothly onto surfaces to ensure both wetting and adhesion. Thus, viscosity as a function of temperature is a key to proper hot melt performance. In addition, factors such as bond strength, flexibility, tack and set time are intimately related to the adhesive’s rheology. By knowing the rheological characteristics of a given hot melt, users can determine its suitability for a given task, or modify the formulation to customize it for a specific application.
One of the best ways to study the rheological behavior of hot melts is through dynamic oscillatory measurements. This technique involves oscillating, or twisting, the material at different frequencies and amplitudes and studying the resultant behavior. Use of this technique enables the user to observe changes in both viscosity and elasticity as a function of temperature or rate — without changing the structure of the adhesive.
Modern oscillatory rheometers, such as the MCR series from Physica (see Figure 1), are ideal tools to study the molecular structure and performance of hot-melt adhesives.
In a typical rheological test, the material is placed between two fixtures (parallel plates in the case of melts, torsion clamps in the case of solids). The test can be performed in one of two ways. The material can be deformed by rotating one of the fixtures by a known amount, and the resulting force is measured. This is known as a controlled strain test. Controlled stress tests can also be performed. These are facilitated by holding the sample in the same manner, but generating data by applying a known force and measuring the resultant deformation. Both methods work well, and instruments such as the MCR can perform either method. The choice of method depends upon the nature of the material and the specific behavior being studied.

Examples of Rheological Measurements
In rheology, we often talk about elasticity, viscosity and modulus.Elasticity can be defined as a material’s ability to store deformational energy, and is represented by G’, or storage modulus. In simple terms, the elastic component of a material can be thought of as a spring; when the deformation is removed, the material uses this energy to return to its original shape.
Viscosity is the measure of a material’s resistance to flow. In oscillatory tests, this can be represented by complex viscosity or h*. Another parameter, G”, or loss modulus, is a measure of the energy lost to the sample during deformation, and is also an indicator of the viscous behavior of a sample. This energy is either used up in the process of changing the sample’s structure, or is dissipated in the form of heat. In oscillatory tests, modulus is represented by G*, and represents the rigidity of a sample, or its “stiffness”.
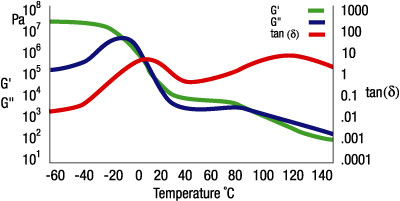
Figure 2 shows typical curves for storage modulus (G’), loss modulus (G”), and loss factor (tan d) for a hot-melt adhesive, measured across a temperature range of -60 to +140 degrees C. At -60 degrees C, the material is an almost rigid solid, with the elastic portion (G’) predominant. The macromolecules of the polymer are “frozen” and immobile.
Above around -20 degrees C, the rubbery region begins and the polymer is getting softer. The G’ values drop, the G” value rises, and both moduli attain the same values at T=0 degrees. Throughout this temperature range the mobility of the molecule increases. At T=8 degrees C, the loss factor attains a maximum value. This peak indicates the glass-transition temperature (Tg). Below the Tg the polymer is in a glass-like state; above the Tg in molten state it has a fluid-like character. Looking at the curves between +20 and +80 degrees C, we see that the G’ once again dominates the material behavior, indicating the presence of superstructures that are not yet completely molten in the polymer. This usually indicates that the amorphous parts of partly crystalline polymers are molten, whereas the crystalline parts are not yet molten. In this case, the more solid crystalline portions are surrounded by the polymer’s liquid amorphous part, like the dispersed component in a dispersion. Since G’>G”, the material still exhibits viscoelastic behavior, with the elastic behavior dominant. Since hot-melt adhesives need to flow freely, this material could cause serious problems if it were used in this temperature range. At temperatures exceeding 80 degrees C, G”>G’ and the fluid character of this viscoelastic melt becomes evident. In this temperature range, the adhesive can be applied more easily, and — because of the relatively low elastic portion — problems such as stringing can be avoided.
Problem Solving through Rheology
The successful application and use of hot-melt adhesives involves many physical properties that can be measured through rheological testing. Flow properties, optimum application temperature and pot life are a few of the areas where rheological testing can be used to analyze and improve a product or process.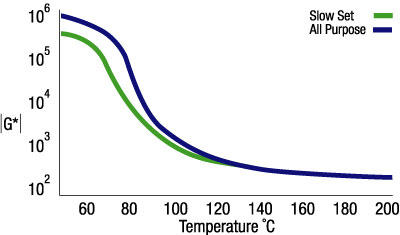
Because resins are often held for several hours in open tanks in dispersing equipment, the viscosity of hot-melt adhesives must be stable over time at processing temperatures. Figure 3 shows viscosity vs. time data for a polyamide resin. Notice how the viscosity drops initially because of molecular weight reduction due to the absorption of water, but over time the viscosity rises slowly. This is caused by slow oxidative crosslinking. A re-formulated resin is also shown that is stable for more than two hours at process temperatures and is better suited to this application.
Additives Can Affect Viscosity
Figure 4 shows viscosity vs. shear rate curves for two hot-melt adhesives: a transparent adhesive and the same material with a pigment added for color. Notice how the hot melt with pigment added has a much steeper slope than the transparent adhesive alone. The addition of pigment has given the adhesive more structure, radically changing its shear behavior. In this case, the process where this adhesive is used will have to be changed to allow for the increased structure of the product with pigment.
Working and Setting Time
The temperature at which an adhesive solidifies can be changed by varying the formulation. Figure 5 shows two hot melts in response to a scan of temperature. One sample is a slow-setting hot melt; the second sample is a multipurpose adhesive. The application temperature for these adhesives is 195 degrees C. At this temperature, the modulus and flow properties of the two adhesives are the same. But as the adhesive cools, it is evident that the slow-set adhesive solidifies at a lower temperature, indicated by an increase in the sample modulus, or G*. By formulating the resin with a lower solidification temperature, the working time of the resin can be increased. The modulus |G*| also provides information about the mechanical stiffness of the adhesive, which relates to the strength of the bond formed between the substrates at the joint interface.