
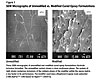
Figure 1.
The epoxies used in these applications are typically one- or two-part systems that offer desirable properties such as adhesion and/or structural strength. The performance properties of epoxy resin formulations are further maximized by the addition of modifiers. These modifiers may improve performance in adhesion, toughness, glass-transition temperature (Tg) or other attributes formulators might need.
Epoxy modifiers come in a variety of forms and chemistries, each offering a different set of benefits - and sometimes challenges. Most conventional modifiers are higher-viscosity liquid or solid polymers that cause further thickening to the uncured epoxy formulation, creating handling challenges. These high formulation viscosities also limit the amount of low-cost fillers that can be used. As a result, the market is showing increased interest in lower-viscosity liquid modifiers that would allow for easier handling and the addition of low-cost fillers without sacrificing performance.
Compatibility can influence the adhesion and/or toughening of the final product. Therefore, it is important to consider compatibility in both the pre-reaction mix and in the final cured epoxy system; some modifiers may be compatible in the pre-reaction mix but may then phase separate upon curing. In addition, others may be incompatible in the pre-reaction mix and stay incompatible upon curing.
An example of a modifier that is compatible in the pre-reaction mix but incompatible (phase separates) during cure is a carboxy-terminated butadiene acrylonitrile copolymer (CTBN) that may or may not be adducted with epoxy resin. CTBN polymers and adducts with bisphenol A epoxy resin serve as industry benchmarks, as they are known for their unique morphology (see Figure 1) and their ultimate performance. The size and shape of the spherical rubbery inclusions that form during the epoxy matrix cure are controlled by the cure kinetics.
A low-molecular-weight reactive diluent, such as an aliphatic polyglycidyl ether or ester, is one modifier that is compatible in both the premix and the cured formula. These materials do not phase separate at any time before or after the epoxy matrix cures, resulting in a transparent, more ductile, or elastomeric epoxy. However, they typically are not used to enhance adhesion or toughness.
Incompatible modifiers, however, do not significantly affect Tgand do not enhance adhesion. On the other hand, they do provide toughening. Examples of modifiers that are not compatible at any time before or after cure are core shell particles or filled or hollow glass beads.
Epoxy formulations can be designed to maximize performance of certain attributes, as desired in a specific final end-use application. However, this is usually accomplished at the expense of other properties. With the exception of incompatible modifiers, adding a modifier usually results in the loss of Tg. Proper formulation is important to achieve balance so that other properties of the epoxies are not compromised in the process to improve a certain performance issue.
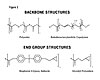
Figure 2.
Study Description
This article examines the structure/property relationship of compatible liquid modifiers. CVC Thermoset Specialties conducted a study to show how variation in modifier molecular weight, backbone composition and end-group functionality affects the modifier and formulation viscosity or handling properties, as well as the Tg, adhesive, and toughness properties of the cured epoxy. As a result of this work, new modifier chemistries have been developed and are documented further in the company’s recent U.S. patent application.Three polymer backbones and two end group functions were used in the study. The polymer backbones were dimer-based polyester, polybutadiene and butadiene/acrylonitrile copolymer. The end group functions were bisphenol A epoxy adducts and glycidyl esters.
All polymer backbones were carboxylic acid terminated. They were then further reacted with either bisphenol A epoxy resin to form adduct esters or with epichlorohydrin to form the glycidyl esters of the carboxylic acids. The bisphenol A epoxy adducts were prepared by reaction of the carboxy-terminated polyesters or butadiene-acrylonitrile copolymers (40 parts). The adducts were prepared with an excess of bisphenol A epoxy resin (60 parts) using triphenylphosphine as a catalyst so that the resulting product contained 40% of the modifier. The glycidyl esters were synthesized by reaction of the carboxy-terminated polyester, CTB or CTBN polymers with epichlorohydrin followed by dehydrochlorination. The structures for the backbones and end-functional groups are shown in Figure 2.
Model Formula and Test Protocol
The model formulation for the study used liquid bisphenol A epoxy resin, dicyandiamide as a curative and OmicureTMU52M as an accelerator. Fumed silica was also added to aid in maintaining the suspension prior to cure. Following is the formulation composition.Bisphenol A liquid epoxy resin
15% modifier based on epoxy resin
6 phr dicyandiamide
3 phr Omicure U52M
2 phr fumed silica
Glass beads (250 micron) were added to maintain constant adhesive thickness in the bond line. All formulation cures were conducted by heating the specimen to 125°C and holding for two hours.
Following are the tests and methods used in this study.
Viscosity: ASTM D 2393
Tgof cured formulation: ASTM E 1356
Lap shear of cured formulation: ASTM D 1002
T-peel of cured formulation: ASTM D 1876
Fracture toughness (K1c) of cured formulation: ASTM D 5045
Formulation preparation included mixing all ingredients together at room temperature using a Cowles mixer. The mixture was then applied to the substrate or poured into a mold. Phosphate-treated, cold-rolled steel (1-by-4-inch) was used as the substrate for all lap shear and T-peel testing. Phosphate-treated, cold-rolled steel was chosen as the test substrate because this substrate is known for providing the best cohesive bond for epoxy adhesives. The goal was to have predominantly adhesive failure to ensure that the properties of the adhesive were truly being measured.

Table 1.
Results and Discussion
Bisphenol A Epoxy Adducted ModifiersFour bisphenol A epoxy adducted modifiers were prepared and tested in this study. Two were based on carboxylic acid terminated dimer polyesters: PriplastTM2101 and 2104. The other two adducts were based on CTBN: HyproTM1300X8 and 1300X13. Each formulation was adjusted such that the modifier content was maintained at 15% of the composition. All samples were cured as stated above.
The viscosities of these modifiers (see Table 1), ranging from about 100,000 to 450,000 cps at 25°C, are inherently higher than the viscosity of the bisphenol A epoxy resin used in the formulation because of the adduction process. The adduction process generates oligomers with the resin yielding higher molecular weight species. The formulation viscosities (observed for Examples 2-5) are also quite high compared to the unmodified Example 1 because of the adducts’ viscosities. The industry benchmark (Example 5) has the highest mix viscosity of those in this study. As bisphenol A epoxy resin adducts are the most widely used modifiers in the industry, this high viscosity has to be accommodated by the use of more specialized and costly formulating process equipment, which limits the amount of low-cost fillers used. Lower-viscosity modifiers are always being requested by the industry.
The Tgof the cured modified formulations (Examples 2-5) is about 15 to 25°C lower than the unmodified formulation. This is due to some level of compatibility of the modifier in the epoxy matrix. Although these adducts are higher in molecular weight, there is a compatibility factor that comes into play because the bisphenol A epoxy moiety is now a part of the molecule enhancing compatibility. Comparison of the Tgof Examples 2-5 shows that Tgincreases with molecular weight but does not appear to be correlated with the polymer backbone composition.
The overall lap shear and T-peel adhesive properties are far better than the standard, and quite similar, with the exception of Example 2, which has a lower molecular weight and glass-transition temperature than the other modifiers in this group. As both polymer backbones are known to exhibit good adhesive properties, these findings are not unexpected. Example 5 exhibits slightly higher adhesive strength, probably because of higher polarity from the increased acrylonitrile content.
Fracture toughness (K1c) properties within the set are good compared to the unmodified formulation. The polyesters of Examples 2 and 3 are better than the CTBN adducts of Examples 4 and 5. The improved K1cin Examples 2 and 3 are indicative of more ductility in the epoxy matrix as a result of more compatibility and lower Tg. Again, there is a relationship showing that molecular weight has an affect on Tg, compatibility and toughness.

Table 2.
Glycidyl ester modifiers were based on Priplast 2104, Hypro 2000X162 and three butadiene/acrylonitrile copolymers: Hypro 1300X31, 1300X8, and 1300X13. These materials, shown in Table 2 as Examples 6-9, have viscosities of ~40,000 to ~576,000 cps at 25°C. These viscosities are lower than viscosities seen for the bisphenol A epoxy adducted materials in Table 1. The mix viscosities of the formulated products also have a significantly lower viscosity than their epoxy adduct counterparts. This is because they are simple glycidyl esters, not oligomeric. Because the entire glycidyl ester molecule is the modifier, these modifiers are used “as is” at 15% loading to achieve the desired modifier level in the epoxy formulation. In contrast, because the modifiers comprise only 40% of the bisphenol A epoxy adduct molecule, the epoxy adducts are used at much higher levels in the formulation to achieve equivalent modifier loading.
A comparison of Tgof Examples 6-10 in Table 2 with the unmodified formulation (Example 1) shows that they are again lower, indicating that there is some portion of the modifier still compatible with the epoxy matrix. However, a comparison of Tgwith the previous study (Table 1) shows that these glycidyl esters are less compatible and have less of an affect on Tg. Again, within the set of Examples 6-10, the Tgfollows along with the concept that higher molecular weight gives higher Tgand less compatibility in the epoxy matrix.
The adhesive properties, as expressed by lap shear and T-peel, are improved over the unmodified formulation in Example 1. The glycidyl butadiene/acrylonitrile copolymers appear to be better than either the glycidyl polyester or the glycidyl polybutadiene. A comparison of the glycidyl esters (Table 2) with the bisphenol A adducts (Table 1) with a similar backbone indicates that the glycidyl esters provide better adhesive properties and higher Tgat lower formulation viscosities.
The glycidyl ester modifiers also show good improvement in fracture toughness compared to the unmodified formulation. The 3,000-molecular-weight glycidyl ester shown in Example 6 exhibits the highest K1cvalue but the lowest Tg. This, again, may be indicative of ductility from some compatibility with the epoxy matrix. The glycidyl polybutadiene ester and all the glycidyl butadiene/acrylonitrile copolymer esters (Examples 7-10) generate similar K1cvalues.

Compact Tension Test Specimens: Thermoset Resin
Summary and Conclusion
The test results demonstrate that the modifiers examined in this study, whether adducted or glycidated, provide significant advantages in performance properties compared to the unmodified control formulation. Results also showed that glycidyl ester modifiers provide significant advantages over the bisphenol A epoxy adducts in their ability to reduce processing viscosity without sacrificing adhesive or toughener performance.Other conclusions of the study include the following.
- The glycidyl ester modifiers are lower in viscosity and yield lower formulation mix viscosities than the industry benchmark.
- Selection of the proper glycidyl ester will allow for higher Tg and desired adhesive and toughener performance.
- The end group functionality appears to affect compatibility in the cured epoxy matrix, which in turn affects Tg. Glycidyl esters exhibit less compatibility than epoxy adducts and thus give higher Tg.
- The study shows that adhesive properties are affected by the polymer backbone. Both polyester backbones and butadiene/acrylonitrile copolymer backbones provide good adhesion.
- The best adhesion performance was achieved with glycidyl ester modifiers prepared using a CTBN.
For more information, contact Dr. Starner by phone at (856) 533-3021 or e-mail Bill.Starner@emeraldmaterials.com.
HyproTM, HyPoxTM, and OmicureTM are trademarks of Emerald Performance Materials companies. PriplastTM is a trademark of Croda Inc.