Additive Advances
We know as consumers to explore the quality of the merchandise we are purchasing to make sure that it not only looks good at the store but also will last as long as intended. As purchasing managers, chemists, production engineers, marketing or business managers, and sales representatives, the products that we purchase, formulate, manufacture, market and sell are required to perform to both internal and external specifications. Ultimately, the products will need to meet the expectations of the consumer. Some of these can be extremely demanding and quality conscious. So, to meet these challenges, we resort to a variety of controls and processes to ensure consistent quality to meet these demands.
With economic factors included in these processes, these challenges are magnified as we face increasing raw materials costs, competition and the omnipresent downward trend in selling prices. As a consequence, cheaper raw materials become increasingly attractive, specifications might be broadened to permit a wider range of conforming materials, and reformulation and material substitutions become more prevalent.
A variety of adhesives are available in today's market. From water- to solventborne, reactive to hot-melt, adhesives are formulated in innumerable ways to meet application requirements. As adhesives technology is dependent upon the use of polymeric, oligomeric and lower molecular weight organic materials, the durability and stability of these materials is dependent not only upon the inherent chemistry and purity of the components but also on the conditions of use. Incorporating functional additives that modify performance and add value to the adhesive is important technology for formulators and manufacturers.
Oxidation of Adhesive Components
Thermal OxidationMany adhesives, as well as the individual constituent materials, are prone to oxidative degradation. Oxidation may occur at relatively low temperatures, including ambient storage. Degradation can occur much more rapidly at elevated use temperatures, as well as during exposure to significantly higher temperatures while mixing or compounding, and during extrusion coating or application to various substrates (as in the case with hot-melt pressure-sensitive adhesives). Hot-melt adhesives are kept at elevated temperatures, often above 300°F, for proper application. As a consequence, hot melts must be sufficiently stable in these temperature ranges either with or without the use of antioxidants. Degradation is initiated by some input of specific energy that may be in the form of either thermal or mechanical energy. Impurities, catalyst residues or an inherently oxidation-prone component can lead to the generation of a free radical species, which then rapidly reacts with oxygen to form peroxy radicals. Peroxy radicals are capable of abstracting hydrogen atoms from somewhere in the adhesive matrix to form hydroperoxides, ROOH, and another free radical, R·. Hydroperoxides are quite unstable and can be easily decomposed into alkoxy, RO·, and hydroxy, ·OH, radicals. These two radicals may then react with other labile hydrogen atoms and continue to propagate more and more radical species. Left unchecked, this cycle can rapidly lead to gross degradation of the adhesive. Fortunately, there are a number of stabilization chemistries that interrupt this auto-oxidation cycle by preferentially and sacrificially reacting with these radical species and hydroperoxides. Stabilizers scavenge these "bad" chemistries and protect the adhesive components from degradation. Of course, the specific antioxidants, concentrations and circumstances in which they are deployed have an important bearing on their effectiveness and adhesive performance. Furthermore, stabilizers are not equally effective at all temperatures. Some stabilizers, such as phosphites and hydroxylamines, are most effective at elevated temperatures and are most commonly used as melt process stabilizers, as their effectiveness is greatest during processing and compounding. Phenolic antioxidants are useful during both processing and as long-term antidegradants.
The mechanism of radical scavenging by phenolic antioxidants is well understood. An oxygen-centered free radical is capable of abstracting the phenolic hydrogen to form the relatively stable phenoxyl radical. This phenoxyl radical is then capable of scavenging additional radicals contributing to the stability of the adhesive. While the phenolic antioxidant plays the role of a sacrificial scavenger, it is chemically transformed by successive radical reactions. Ultimately, if the radical generating mechanisms are too great, the transformation of the antioxidant can lead to the generation of a chromophore, which can lead to noticeable discoloration of the material. Although this discoloration may be undesirable, in the absence of a stabilizer the adhesive may discolor at an even faster rate and/or suffer from loss of adhesive properties with the possibility of product failure. Optimized antioxidant formulations can provide significant improvements in adhesives formulations, and can often times avoid unwanted discoloration.
The use of secondary antioxidants, such as phosphites and thioesters, can often improve stabilization during extrusion and compounding, and may also provide improvements in color. Phosphites decompose hydroperoxides into non-radical generating byproducts. Thioesters are typically used in combination with phenolic antioxidants. Thioesters are commonly used in adhesives requiring long-term, high-temperature performance. Ciba® IRGANOX® PS 800 is a traditional thioester. Diphenylamine based stabilizers, such as IRGANOX 5057, are also commonly used in hot-melt adhesive formulations as secondary stabilizers. Diphenylamines, when used in combination with phenolic antioxidants, also provide excellent long-term thermal aging while maintaining good color control. Hydroxylamine antioxidants are multifunctional and can react with both radicals as well as hydroperoxides. Furthermore, as phenolic antioxidants can discolor in the presence of oxides of nitrogen, in so-called gas fade reactions the use of a the hydroxylamine Ciba IRGASTAB® FS 042 has demonstrated effectiveness in diminishing the severity of these reactions, and when used in place of phenolic antioxidants, can largely eliminate gas fade discoloration. Hydroxylamine technology, however, is not a direct drop-in alternative to phenolic antioxidants, so a total stabilization concept needs to be developed when using hydroxylamine. A common co-stabilizer class used with hydroxylamine to provide long-term antioxidant performance is the hindered amine stabilizer. Ciba CHIMASSORB® 944 is representative of this class and is used successfully in several polymer applications in conjunction with IRGASTAB FS 042. A phosphite such as Ciba IRGAFOS® 168 is also commonly formulated to provide additional processing stability.
Benzofuranones are a more recent addition to the antioxidant arsenal. These extremely potent radical scavengers are capable of reacting directly with carbon-centered radicals. Consequently, they can interrupt the auto-oxidation cycle earlier than phenolic and phosphite type stabilizers. Due to their effectiveness at low concentrations, they are typically formulated as a minor component in combination with phenol-phosphite blends. Combinations of HP 136 with IRGANOX 1010 and IRGAFOS 168 are most commonly formulated. Additionally, HP 136 is able to provide protection to the polymer in inert atmospheres, whereas traditional antioxidants require the presence of oxygen to be effective.
Photo-Oxidation
Although many adhesive compositions are sensitive to photo-oxidation, most adhesive applications do not require any significant ultraviolet (UV) light stabilization. There are, however, some applications where UV exposure is a valid concern and special attention and formulations are required. For example, certain labeling applications may be subjected to UV exposure. Applications might include decorations or labels used in outdoor applications. Safety labels applied to equipment, machines, electrical cabinets and telecommunication pedestals all contain labels warning of potential dangers. Transparent packaging labels, which are exposed to lesser UV levels, may also be prone to UV degradation and can discolor over time. Two classes of stabilizers are frequently used to provide UV protection: ultraviolet light absorbers (UVA) and hindered amine light stabilizers (HALS). UVAs function by absorbing UV light and converting this UV energy into molecular vibrations. This absorption mechanism follows Beer's law, so path length and additive concentration are important in establishing effective formulations. In contrast, HALS can be very effective as radical scavengers, and are not subject to Beer's law constraints. HALS are much more effective than other radical scavengers in providing stability in photo-oxidative environments. In comparison, phenolic antioxidants are relatively ineffective as UV stabilizers. In many adhesive applications where UV stabilization is required, both HALS and UVAs are used for stabilization.Examples of Thermal Stabilizers in Hot-Melt Adhesives
Since adhesives are multicomponent systems, one needs to consider how the individual components will perform as part of the more complex formulated system. One means of determining the suitability of a candidate component is to understand how it might perform individually, in isolation. In the context of stability and durability, how susceptible is the component to degradation, and how effectively can the material be improved by the judicious use of antioxidants? In other instances, it is more practical to evaluate the effectiveness of stabilizers in fully formulated systems.APAO-Based Hot-Melt Adhesives
Amorphous polyalpha olefins (APAO) are a family of olefin-based thermoplastic polymers offering a combination of both physical and chemical properties. The advantages of low molecular weight and low crystallinity (high amorphous level) provide APAOs with characteristics such as easy processing, controlled tack and adhesive strength, and low temperature flexibility, as well as low density and excellent moisture resistance. Antioxidants are important in improving and maintaining the thermal stability of neat APAOs for both viscosity and color control. While phenolic antioxidants provide very good viscosity control, the addition of a secondary stabilizer, such as a phosphite or thioester, can provide additional improvements with regard to color while still maintaining good viscosity control.
SBC-Based Hot-Melt Adhesives
Styrenic block copolymers are designed for use in a variety of adhesive applications including personal care disposables, tapes, labels, and construction adhesives. The hard styrenic end blocks and soft elastomeric center blocks of these copolymers phase separate over wide end-use temperature ranges and make them ideal formulating components for hot-melt adhesives. Neat SBCs typically contain a base antioxidant system added by the manufacturer. However, for many HMA applications, additional antioxidant is required to meet the processing stability and long-term thermal aging requirements of the application.
Styrene-isoprene-styrene (SIS) and styrene-butadiene-styrene (SBS) block copolymers, due to the unsaturation in the mid-block, are inherently more prone to oxidation than the fully saturated styrene-ethylene-butylene-styrene (SEBS) block copolymers. Regardless of inherent stability, these materials are widely used due to the beneficial attributes and formulating flexibility contributed by the differing chemical compositions.
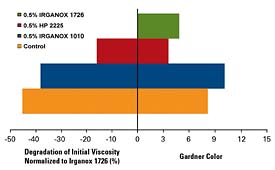
Figure 1 shows the Gardner color and melt viscosity performance for several different antioxidant systems. Ciba IRGANOX 1010 is the workhorse antioxidant in the adhesives industry, with good all-around performance attributes. IRGANOX 1726, a new multifunctional antioxidant at an equal weight loading, demonstrates improved performance in both maintaining color and viscosity. Since these are fully formulated systems, the antioxidant demonstrates effectiveness not only in stabilizing the polymer, but also in stabilizing the tackifiers.
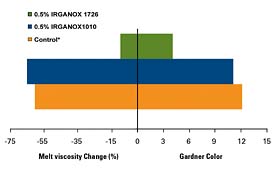
IRGANOX 1726 again has been found to provide significant improvements in HMA type applications using SBS. Figure 2 illustrates the improved performance of IRGANOX 1726 vs. the more traditional IRGANOX 1010 and 1010/IRGANOX PS 800 blend systems. The data demonstrates that both the color and the viscosity are better controlled with IRGANOX 1726.
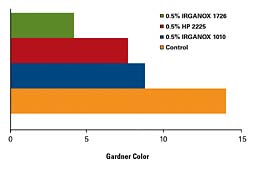
Although SEBS is inherently more stable than either SBS or SIS, it too requires an appropriate stabilization system. Figure 3 illustrates the benefits of a well-designed stabilizer package, IRGANOX HP 2225. Improvements in color, as well as retarding changes in viscosity and postponing skin formation, are attributes commonly seen with this stabilizer package.
EVA-Based Hot-Melt Adhesives
EVA is another widely used polymer for hot-melt adhesives. Applications include labels, case and carton closing, laminating, bookbinding, and product assembly, as well as uses in the ubiquitous glue-gun glue-stick. Vinyl acetate levels can range from a few percent to 40%. Low melt index grades provide high viscosity, strength and hot tack. In contrast, high MI grades enable higher polymer content with low application viscosities. Mid-range MI grades provide formulation flexibility. Antioxidant evaluations show that a phenolic antioxidant, phosphite and a benzofuranone combination is very effective at maintaining the adhesive properties during aging, and minimizes color development. A common blend with these components used in the adhesive industry is IRGANOX HP 2225.
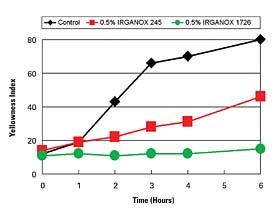
Hot-melt polyurethane (HMPUR) adhesives are used in a number of applications. HMPUR adhesives application temperatures range from 85°C to 140°C (185-284°F), which is less than that of some other types of thermoplastic hot-melt products. Stabilization of PUR compounds can benefit from the use of different antioxidants than encountered in olefin and styrenic-based materials. Figure 4 demonstrates the performance advantages of a IRGANOX 1726 vs. a commonly used product, IRGANOX 245. 1726 is a liquid at room temperature, whereas 245 is a solid. The liquid form of IRGANOX 1726 is a product attribute that is often favored by the PUR industry.
Application of Antimicrobial Additives for Hot-Melt Adhesives
Microorganisms, such as bacteria, algae and fungi, can colonize, reproduce, and attack their host surfaces, which can result in discoloration, staining, odor, bio-film formation, cross-contamination, and eventually deterioration of mechanical properties and reduced service life of the finished product. Any of these occurrences can be considered a product quality issue. A variety of antimicrobial additives are available that can be considered for adhesive applications depending upon the specific effect and attributes required. As these products are highly regulated by the U.S. Federal Government, it is important to work closely with suppliers to ensure regulatory compliance.Ciba IRGAGUARD® B 1000 exhibits antimicrobial activity against a wide spectrum of bacteria. The combination of low migration rates and high activity of B 1000 generally provides antimicrobial efficacy over the entire life of the polymer article. The balance between substrate compatibility and migration rates makes this product suitable for many common substrates, including hot-melt adhesives.
In another example, IRGAGUARD F 3000 was evaluated in a mattress adhesive ticking application. The mattress fabrics were exposed to a fungus found in mattresses. Results indicated the treated mattress adhesives significantly inhibited fungal growth. In addition, F 3000 was determined to have no adverse effect on the color or adhesive properties (tack) of the hot-melt adhesive during extended aging of the adhesive.
Summary
Hot-melt adhesives are complex materials that are used in a variety of end-use applications. Performance requirements vary considerably from one application to the next, and a multitude of formulations have been developed to meet these varying requirements. In addition to polymers, tackifiers, waxes and oils, functional additives are important formulating materials for hot melts. Antioxidants prevent degradation of adhesive components and systems, and contribute greatly to initial quality and long-term performance. UV stabilizers are specialty additives that provide enhanced durability and resistance to discoloration for adhesives exposed to the damaging effects of UV light from both natural (sunlight) and artificial light sources. Antimicrobial products can be formulated into adhesives for applications in which bacteria, fungi and algae growth adversely affect product performance and suitability for use. Each of these technologies enables hot-melt adhesives to be formulated for new and ever-more demanding applications.This article is based on a paper presented at the TAPPI 2003 PLACE Conference and Global Hot Melts Symposium.
IRGANOX®, IRGAFOS®, TINUVIN®, CHIMASSORB®, IRGAGUARD®, and IRGASTAB® are registered trademarks of Ciba Specialty Chemicals Corp.
For more information, contact Ciba Specialty Chemicals, 540 White Plains Road, Tarrytown, NY 10591.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




