Hydroxyl-Terminated Polybutadiene Liquid Resins for Adhesives Applications
Hydroxyl-terminated polybutadiene resin-based polyurethane elastomers offer solutions to strenuous applications in the sealants and adhesives market.




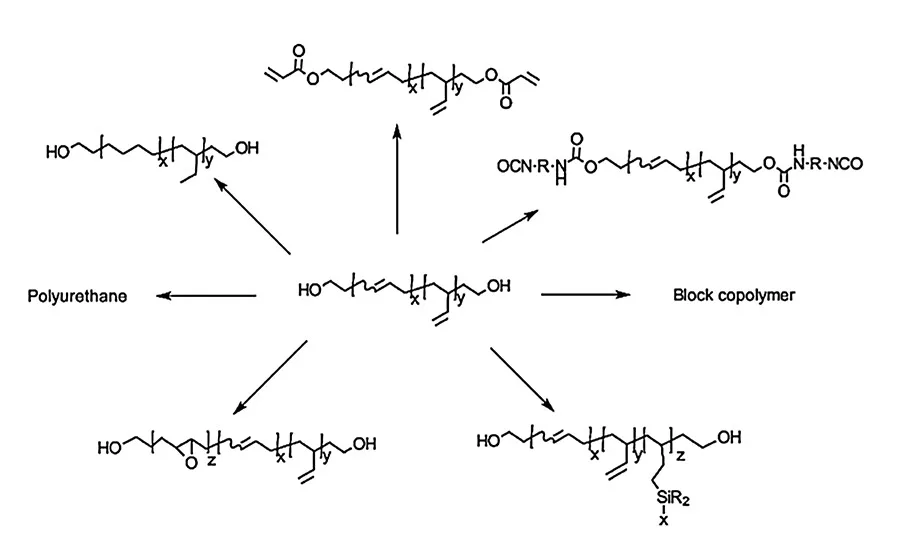
Figure 1. Chemical modification of HTPB.


Hydroxyl-terminated polybutadiene (HTPB) resins occupy a unique space within the landscape of commercially available polyols in the context of polyurethane elastomers. Characterized by low temperature flexibility, hydrophobicity and hydrolytic stability, polyurethane elastomers present solutions to strenuous applications in the adhesives and sealants market. Due to the hydrocarbon nature of the polymer backbone, these elastomers also have extremely low dielectric constant, dissipation factor, and moisture vapor transmission rate (MVTR) properties.
With the fast growth of the information industry, electronic products such as mobile phones, personal digital assistants and notebooks have become daily necessities for many of us. Multifunctional urethane (meth)acrylates derived from hydrogenated HTPB resins, which have excellent properties against oxidation and a relatively high refractive index of about 1.5, have gradually established their use as essential ingredients in UV-cured compositions in a clear optical index-matched adhesive for the bonding of two or more optical components together.
Background
HTPB is a telechelic polymer having two or more reactive hydroxyl end groups. Commonly, HTPB telechelic polymers are prepared by two different polymerization technologies: free radical and anionic polymerization. The chemical structure and architecture of this polymer depends upon the method of synthesis and polymerization reaction condition.1
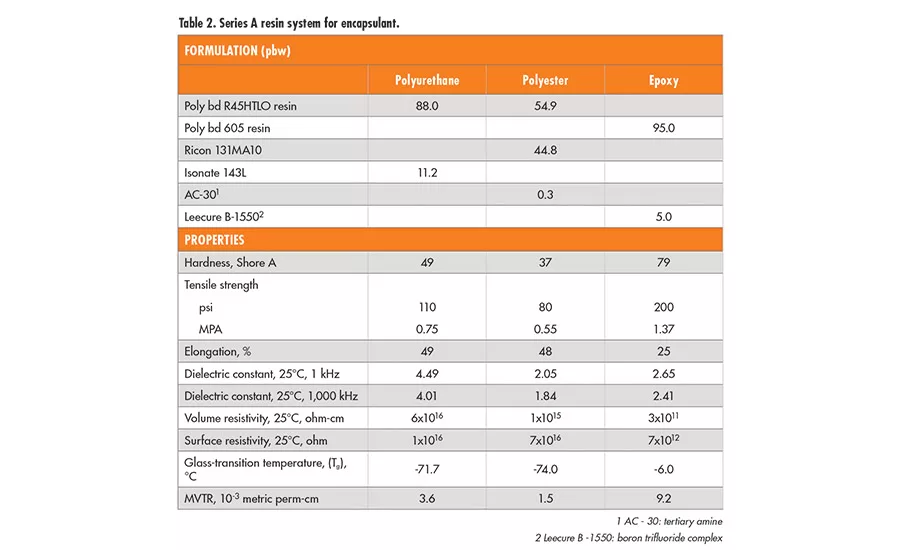
A series of high-performance hydroxyl-terminated polybutadiene liquid resins* (series A resins) is used worldwide in adhesives, sealants, and electrical applications due to their low glass-transition (Tg) temperatures (-75°C), hydrophobicity, acid-base resistance, and excellent dielectric properties. These liquid polybutadiene resins are made by radical polymerization using hydrogen peroxide initiators and have only about 20% 1,2-vinyl moiety. Although these resins have a predominantly linear backbone structure, they also contain a portion of branched polymers resulting from the radical polymerization process. Thus, the commercial product has a hydroxyl functionality of 2.4-2.6 per chain on average. This branched structure occasionally imposes limitations on customers’ processes or product performance, especially when they seek design flexibility between thermoplastic and thermoset properties.
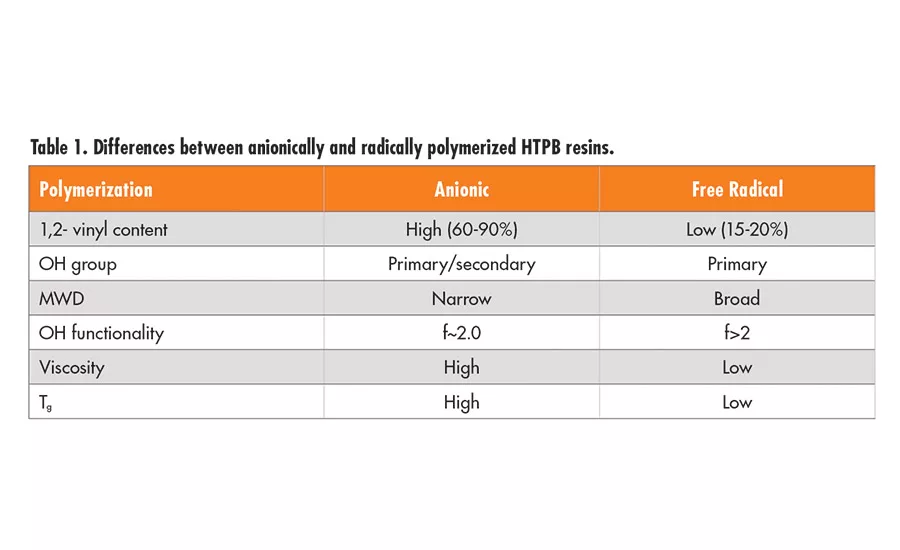
In addition, another series of HTPBs** (series B resins) with lower average hydroxyl functionality is available. These LBH (secondary alcohol termination) and LBH-P (primary alcohol termination) resins are linear diols with an average of 1.95 hydroxyl groups per chain and no species with functionality higher than two. They are made by anionic polymerization and have approximately 65% 1,2-vinyl structure. These difunctional resins can be used to prepare thermoplastic polyurethane elastomers. Depending on the hydroxyl functionality and backbone microstructure, namely, the 1,2-vinyl, 1,4-cis, or 1,4-trans distribution of each repeating unit, the HTPB-derived polyurethane elastomers can have a wide range of physical properties, including Tg.2,3 The differences between the HTPB polymers produced by anionic and free radical polymerizations are shown in Table 1.
HTPBs can be chemically modified through various chemical reactions. For example, polyols like HTPBs are used for making polyurethanes with chain extenders. The OH group at the chain end can be converted to various functional groups like acrylate, isocyanate, and carboxylic acid. It can also be used as an initiator for making block copolymers with ring opening polymerization. During the last several decades, interest has grown in the functionalization/chemical modification of HTPBs by grafting different compounds onto the unsaturation of the main chain, such as epoxidation, or pendant vinyl groups, such as hydrosilylation and thiol-ene reaction. They provide a means for altering the physical and chemical properties of the polymer.1,4-6
Hydrogenation is another important method for polymer modification, leading to a variety of useful elastomers and thermoplastics with unique structures and properties.7 Hydrogenation also offers a convenient synthetic route to polymers with unique monomer sequences. For example, polybutadiene can be hydrogenated to form copolymers of ethylene with various amount of 1-butene. The resulting hydrogenated rubber has good resistance to oxidative and thermal degradation, improved weathering, excellent chemical resistance at elevated temperatures, low permeability to gases, and better processability when compared to the unmodified elastomers. Hydrogenated hydroxyl-terminated polybutadiens are becoming increasingly important in the composition of adhesives and sealants because of their superior thermal and light stability compared to their unsaturated precursors. For instance, the acrylated urethane oligomers derived from hydrogenated HTPBs are used for adhesives in touch panels to increase the color clearance due to excellent light stability.
Results and Discussions Encapsulants
From the 1950s, epoxy resins became available and were used due to their high strength, chemical resistance, and excellent adhesion. Silicones and polysulfides were also used as encapsulants from this date.8 Polyurethanes are also electrical insulators, and their use as potting or casting resins for the protection of electrical and electronic parts has grown substantially over the years. Since their electrical properties are comparable to the epoxy resins, they can be used in similar applications. Compared with epoxy resin encapsulation, polyurethane encapsulation remains soft over a wide temperature range, and inductance shift is reduced to the extent that no compensation of the winding is necessary in telephone line loading coils.9
Encapsulants based on polybutadiene polyols have proven their superiority for applications in which the combination of resistance to temperature shock and very low moisture absorption is required. Due to their very low glass-transition temperature (-70°C), no internal stress is generated over a wide temperature range. This is an important feature for pressure-sensitive electronic components. A quantitative comparison of electronic component/solder joint stress relief in encapsulated assemblies was reported.10
The majority of the polyurethane encapsulant formulations are based on MDI. Dependent on the final application, this can range from polymeric MDI, with or without modification, to prepolymers based on pure MDI with varying degrees of functionality. The addition of the lower reactivity 2,4-MDI is used to control the reaction rate, the viscosity buildup and to reduce the exotherm.
Hydrolytic Stability
Formulations based on series A resins are known for their excellent hydrolytic stability and low moisture permeability. Measuring moisture vapor transmission rates (MVTR) is one method of determining the permeability of a material. Figure 2 shows the MVTR of a variety of series A resin-based systems in comparison to other typical electrical potting and encapsulation compounds. The MVTR of series A resin-based formulations are competitive, if not superior to the MVTR exhibited by other commonly used electrical potting and encapsulation materials.
Thermal Shock/Embedment Stress
Series A resin-based elastomers maintain essentially constant elongation and low embedment stress over a wide range of temperatures. This feature allows encapsulation of fragile electrical components in a relatively stress-free environment, even at low temperatures. Series A resins can be formulated into systems exhibiting excellent thermal cycling properties in potting or encapsulation applications. Even at -38°C, embedment stress is significantly lower than that of most competitive materials.
Low Exotherm and Minimal Shrinkage
A variety of series A resin-based systems cure at ambient temperature with minimal exotherm. Series A resins react with isocyanates at ambient temperature. Pot life and cure time can be adjusted using standard urethane catalysts. Series A resins also react with maleic anhydride adducted polybutadiene to produce esters. Tertiary amine catalysts can be used to achieve ambient temperature cures. Series A resin systems exhibit minimal shrinkage after curing.
Insulating Glass Window Sealants
Series A resin-based sealants are used worldwide as engineering materials in marine, automotive, building construction and, especially, insulating glass window applications. Sealants for insulating glass windows must meet the highest standards of performance. Urethane-cured series A resin-based formulations are capable of providing excellence in adhesion, UV exposure resistance, and physical properties over a wide temperature range. These advantages are due to the ability of series A resins to impart excellent:
• Adhesion to metals and glass
• Low temperature flexibility
• Water resistance
• Property retention with age
• Dispersion of fillers
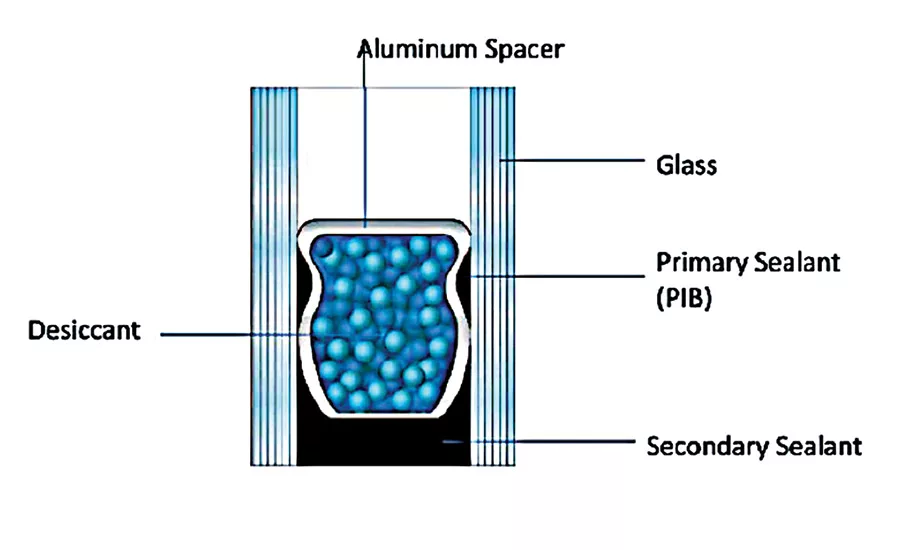
According to current standards, elastically sealed insulating glass units (IGUs) are double sealed: the inner or primary sealant is mainly thermoplastic polyisobutylene (PIB) or butyl rubber, which is applied to reduce the water vapor and gas permeability of the edge seal but also acts as processing aid to hold the spacer bar in place during manufacture. The outer or secondary sealant functions as an adhesive, which holds the unit together and restricts moisture transmission into the unit and gas permeation out of the unit. The desiccant in the spacer reduces the dew point of the air in the spacer between the two panes. This prevents condensation on the interior side of the exterior pane of glass. Because it is exposed to low temperatures, the sealant must have a low glass-transition temperature. Secondary sealants are based on different materials—the most important are polysulfide (PS), polyurethane (PUR) and silicone polymers (SI). A starting point formulation using series A resin R-45HTLO as the base resin for an insulating glass window sealant is shown in Table 3.
Liquid Optically Clear Adhesive
Liquid optically clear adhesive11,12 (LOCA) is a relatively new technology in display bonding. Coexisting with traditional optically clear adhesive (OCA) tapes and films, a LOCA has been used to attach the components within a display device between a cover lens or touch sensor and the liquid crystal display (LCD) module to enhance the viewing experience by significantly improving the contrast.
LOCA offers many advantages over OCA tape and is becoming an attractive solution for assembling display modules. As the number of devices using touch panels has increased exponentially, the market for projected capacitive touch panels has also expanded. Polybutadiene or hydrogenated polybutadiene has a relatively high refractive index of about 1.5, which is very close to that of glass used in touch panels. Multifunctional urethane (meth)acrylates derived from hydrogenated polybutadiene diol resins have gradually established their utility as essential ingredients in UV-cured LOCA composition.
A resin composition based on hydrogenated polybutadiene resin, which has been in high demand in LOCA applications, is likely to be a urethane (meth)acrylate derivative. It is made by reacting hydrogenated polybutadiene diol with the aliphatic/aromatic diisocyanate to form isocyanate-terminated prepolymer, and then followed by capping the isocyanate functionality with hydroxyl-functionalized (meth)acrylate, such as 2-hydroxyethyl methacrylate (HEMA). The composition appears to be non-proprietary, as many issued patents and patent applications have employed this intermediate for specific application.
A recent LOCA composition comprises: a) a (meth)acrylate oligomer having one or more functional groups; b) a mono-functional monomer, a multifunctional monomer, or a mixture thereof; c) a photoinitiator; and d) a plasticizer having a refractive index of no less than 1.48. For example, the multifunctional (meth)acrylate oligomer may comprise a multifunctional urethane (meth)acrylate oligomer having at least two (meth)acrylate groups (e.g., from two to four (meth)acrylate groups) that participate in polymerization during curing. In general, these oligomers comprise the reaction product of a polyol with a multifunctional isocyanate, followed by termination with a hydroxyl-functionalized (meth)acrylate.13
Ideal Ingredients
Hydroxyl-terminated polybutadiene resin-based polyurethane elastomers characterized by low temperature flexibility, hydrophobicity, and hydrolytic stability present solutions to strenuous applications in the sealants and adhesives market. Due to the hydrocarbon nature of the polymer backbone, these elastomers also possess extremely low dielectric constant, dissipation factor, and MVTR properties.
With the fast growth of the information industry, electronic products such as mobile phones, personal digital assistants, and notebooks have become daily necessities in the life of modern-day individuals. Multifunctional urethane (meth)acrylates derived from hydrogenated HTPB resins, which have excellent properties against oxidation and a relatively high refractive index of about 1.5, have gradually established their utility as essential ingredients in UV-cured composition for the optical bonding of two or more optical components together using a clear optical index-matched adhesive. ASI
For more information, email ext-techsupport@total.com or visit www.crayvalley.com/home/ASI2017.
References
1. P. Santhana Gopala Krishnan, Kavitha Ayyaswamy and S.K. Nayak, J. Macromol. Sci., Pure Appl. Chem. (2013) 50, 128-138.
2. A. Dey1, M.A.S. Khan, J.Athar, A.K. Sikder1 and S. Chattopadhyay, J. Mater. Sci. Eng, B 5 (3-4) (2015) 145-151.
3. Z. Cao, Q. Zhou, S. Jie, and Bo-Geng LiInd, Eng. Chem. Res, (2016) 55, 1582-1589.
4. Q. Wang, X. Zhang, L. Wang, Z. Mi, J. Mol. Catal. A, Chem, (2009) 309, 89-94.
5. O. Mykhaylyk, C. Fernyhough , M. Okura, J. Fairclough, A. Ryan, R. Graham, European Polymer J. (2011) 4, 447-464.
6. G. Boutevin, B. Ameduri, B. Boutevin, J. Joubert, J. Appl. Polym. Sci., (2000) 75, 1655-1666.
7. N. K. Singha, S. Bhattacharjee and S. Sivaram, Rubber Chem. Technol, 70, 309 (1997).
8. A. Worley, J. Elastoplastics, (1969), 201-7.
9. M. Szymanski, A. Worley, Western Electric Engineer, (1973) 17(2), 19-23.
10. D. Cummings, Report (1979), BDX-613-2185, CONF-790506-4, 8 pp. Avail.: NTIS From: Energy Res. Abstr. 1979, 4(13), Abstr. No. 37132.
11. US2012/0172477 A1: C. Huang, C. Lu and H. Shih, 2012.
12. B. Bahadur, J. Sampica, J. Tchon, and A. Butterfield, J. Soc. Information Display, Vol. 19, 11, 732–740, November 2011.
13. US 2013/0011683 A1, S. Busman, S. Pillalamarri, T. Tran, C. Campbell, 2013.
*TOTAL Cray Valley Poly bd® resins
**TOTAL Cray Valley Krasol® resins
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





.webp?height=200&t=1685029884&width=200)


