Managing the Concerns of BPA
A new perspective on managing bisphenol A content and seeking out alternatives.

 Bisphenol
A (BPA) is a chemical that has recently received a lot of media attention,
primarily due to a report citing its possible estrogenic effects. This report,
published by the Center for the Evaluation of Risks to Human Reproduction
(CERHR), has raised concerns with manufacturers and consumers who encounter
BPA, which is used directly in polycarbonate plastics, epoxy resins and - as
greatly publicized by the media - some plastic water and baby bottles. BPA can
also be encountered indirectly as a manufacturing byproduct present in many
other chemicals, including (bisGMA), ethoxylated (N) bisphenol A Diacrylate
(EBPADA) and ethoxylated (N) bisphenol A Dimethacrylate (EBPADMA). These
chemicals are low-volatility monomers commonly used in free-radical
polymerizations. They are also used in applications such as adhesives,
coatings, sealants, elastomers, plastisols and photopolymers. Despite its
recent negative attention, BPA may not necessarily need to be completely
eliminated from formulations. Many of BPA’s direct applications involve minimal
risk of human ingestion, such as the manufacture of CDs or car headlight
covers. Where BPA is a concern, alternative BPA-free materials are available
for applications like epoxy coatings, dental composites and sealants that pose
a direct risk of human ingestion.
Bisphenol
A (BPA) is a chemical that has recently received a lot of media attention,
primarily due to a report citing its possible estrogenic effects. This report,
published by the Center for the Evaluation of Risks to Human Reproduction
(CERHR), has raised concerns with manufacturers and consumers who encounter
BPA, which is used directly in polycarbonate plastics, epoxy resins and - as
greatly publicized by the media - some plastic water and baby bottles. BPA can
also be encountered indirectly as a manufacturing byproduct present in many
other chemicals, including (bisGMA), ethoxylated (N) bisphenol A Diacrylate
(EBPADA) and ethoxylated (N) bisphenol A Dimethacrylate (EBPADMA). These
chemicals are low-volatility monomers commonly used in free-radical
polymerizations. They are also used in applications such as adhesives,
coatings, sealants, elastomers, plastisols and photopolymers. Despite its
recent negative attention, BPA may not necessarily need to be completely
eliminated from formulations. Many of BPA’s direct applications involve minimal
risk of human ingestion, such as the manufacture of CDs or car headlight
covers. Where BPA is a concern, alternative BPA-free materials are available
for applications like epoxy coatings, dental composites and sealants that pose
a direct risk of human ingestion.
CERHR has focused on BPA because of its widespread uses and, in some cases, direct exposure to humans. The chemical nature of BPA - specifically its two phenol groups - has lead researchers to believe that it has potential for interaction with biological active sites. Exposure to BPA can occur through inhalation or ingestion. Those formulating or handling BPA directly have the highest inhalation risk. Studies have shown that the release of BPA from products like epoxy liners is dependent upon temperature. Heat and age can both increase BPA migration.
The result of BPA exposure has been actively debated. The National Toxicology Program, an office within the National Institutes of Health, acknowledged in a draft report that the chemical might cause cancer and other serious disorders. However, further studies conducted by the U.S. National Toxicology Program have shown that there is insufficient evidence to state that BPA is carcinogenic or mutagenic. Groups directly related to the manufacture and sale of BPA claim that current exposure levels are well below permissible limits. They also cite reports that refute the claims of the CERHR. Studies by the European Union show that BPA exposure at “realistic” doses does not have any effect on reproduction or development. With the debate over the safety of BPA ongoing, it would be wise to carefully examine applications involving direct contact with food and drink or medical devices.

 Other options are available for formulators who like the
attributes of BPA-containing materials but are concerned over its residual
content in their final product. The commonly used ethoxylated (N) bisphenol A
Diacrylate (EBPADA) and ethoxylated (N) bisphenol A Dimethacrylate (EBPADMA)
can have levels of BPA ranging from approximately 20 ppm to 700 ppm, depending
upon their level of ethoxylation. In general, the higher the level of
ethoxylation, the lower the concentration of BPA in EBPADMA. Examples of these
BPA ranges can be found in Table 2. Replacing the 2-4 ethoxylated EBPADMA with
a higher-ethoxylated EBPADMA will reduce BPA content, but it will also affect
the physical properties of the final polymer, such as crosslink density, degree
of conversion, water sorption and flexural strength. Blending different
EBPADMAs with bisGMA can also lower BPA levels. Urethane dimethacrylates are
another common class of alternatives. Custom variations upon a urethane
dimethacrylate make it possible to mimic the desired chemical or physical
properties of BPA-containing resins, such as toughness, optical clarity, high
heat resistance, and good electrical resistance.
Other options are available for formulators who like the
attributes of BPA-containing materials but are concerned over its residual
content in their final product. The commonly used ethoxylated (N) bisphenol A
Diacrylate (EBPADA) and ethoxylated (N) bisphenol A Dimethacrylate (EBPADMA)
can have levels of BPA ranging from approximately 20 ppm to 700 ppm, depending
upon their level of ethoxylation. In general, the higher the level of
ethoxylation, the lower the concentration of BPA in EBPADMA. Examples of these
BPA ranges can be found in Table 2. Replacing the 2-4 ethoxylated EBPADMA with
a higher-ethoxylated EBPADMA will reduce BPA content, but it will also affect
the physical properties of the final polymer, such as crosslink density, degree
of conversion, water sorption and flexural strength. Blending different
EBPADMAs with bisGMA can also lower BPA levels. Urethane dimethacrylates are
another common class of alternatives. Custom variations upon a urethane
dimethacrylate make it possible to mimic the desired chemical or physical
properties of BPA-containing resins, such as toughness, optical clarity, high
heat resistance, and good electrical resistance.
 Many
industries have been affected by the safety concerns involving BPA, including
the dental industry. The dental materials industry has calmed BPA concerns
among their final consumers by being very careful when selecting their raw
materials and by using traditional BPA-free adhesive systems.
Many
industries have been affected by the safety concerns involving BPA, including
the dental industry. The dental materials industry has calmed BPA concerns
among their final consumers by being very careful when selecting their raw
materials and by using traditional BPA-free adhesive systems.
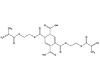
 These adhesive systems have potential applications in other
industries, including the NTG-GMA/PMDM system. PMDM is pyromellitic
dimethacrylate (see Figure 2) and NTG-GMA is a surface-active co-monomer of N
(p-tolyl) glycine and glycidyl methacrylate (see Figure 3). Together, they
create an effective adhesive system capable of bonding to various
surfaces. The NTG-GMA/PMDM system is
commonly delivered to a bonding site in a solution of acetone, ethanol or
hydroxyethyl methacrylate (HEMA). Both PMDM and NTG-GMA are TSCA-listed and
approved for all industrial applications. In the mouth, they provide an
excellent way of bridging the gap between the hydrophilic dentin and the
hydrophobic composite. They could play the same role in industrial settings
when a bond is needed between differing substrates with material that is
BPA-free.
These adhesive systems have potential applications in other
industries, including the NTG-GMA/PMDM system. PMDM is pyromellitic
dimethacrylate (see Figure 2) and NTG-GMA is a surface-active co-monomer of N
(p-tolyl) glycine and glycidyl methacrylate (see Figure 3). Together, they
create an effective adhesive system capable of bonding to various
surfaces. The NTG-GMA/PMDM system is
commonly delivered to a bonding site in a solution of acetone, ethanol or
hydroxyethyl methacrylate (HEMA). Both PMDM and NTG-GMA are TSCA-listed and
approved for all industrial applications. In the mouth, they provide an
excellent way of bridging the gap between the hydrophilic dentin and the
hydrophobic composite. They could play the same role in industrial settings
when a bond is needed between differing substrates with material that is
BPA-free.
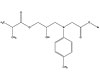
 Pyromellitic glycerol dimethacrylate, or PMGDM, is another
BPA-free alternative monomer (see Figure 4). In the dental industry, it is
often used in a single-solution formulation with a photoinitiator. The chemical
structure of PMGDM enables it to bond two surfaces of differing polarity. Its
acid groups gravitate toward bonding with polar substrates, leaving its
dimethacrylate groups available to bond to other less-polar or hydrophobic
sites. PMGDM commonly uses acetone or TEGDMA as a solvent.
Pyromellitic glycerol dimethacrylate, or PMGDM, is another
BPA-free alternative monomer (see Figure 4). In the dental industry, it is
often used in a single-solution formulation with a photoinitiator. The chemical
structure of PMGDM enables it to bond two surfaces of differing polarity. Its
acid groups gravitate toward bonding with polar substrates, leaving its
dimethacrylate groups available to bond to other less-polar or hydrophobic
sites. PMGDM commonly uses acetone or TEGDMA as a solvent.
Glass ionomer cements (GIC) are common BPA-free adhesives used in the dental industry. These cements are two-component systems comprised of a mixture of polymerized acids and specifically designed glass powder. When mixed, the acids begin to partially dissolve the glass particles. The acid polymers then combine chemically with the dissolved glass components to produce a hard matrix material. The final matrix is reasonably translucent, allowing for various formulator manipulations to occur. GICs work well in moist environments, and are good for bonding ceramics to metals. In the dental industry, GICs are also used to bond gold and base metals to resin composites. Their utility allows them to be applied directly to clean substrates without additional preparation.
Research and development has focused on creating resin-modified glass ionomer cements. Resin-modified GICs are still considered BPA-free and add an additional method of curing. Chemists have created an adhesive system with increased toughness that can be chemically cured or photo-cured by introducing resins to GICs.
The ongoing debate over the risks associated with BPA can make it difficult to set a clear course of action. Those handling BPA directly or indirectly should ask themselves the following questions.
For more information, contact Esstech Inc., 48 Powhattan Ave., P.O. Box 39, Essington, PA 19029; phone (610) 521-3800 or (800) 245-3800; fax (610) 521-4600; e-mail techsupport@esstechinc.com; or visit www.esstechinc.com.

BPA is used directly in polycarbonate plastics, epoxy resins
and - as greatly publicized by the media - some plastic water bottles.

Figure 1. BPA
What is BPA?
BPA, or bisphenol A (see Figure 1), is an organic compound that makes up the backbone of many polycarbonate polymers. It has also been used as a plasticizer in PVCs. BPA adds clarity and toughness to epoxies, coatings, and adhesives.CERHR has focused on BPA because of its widespread uses and, in some cases, direct exposure to humans. The chemical nature of BPA - specifically its two phenol groups - has lead researchers to believe that it has potential for interaction with biological active sites. Exposure to BPA can occur through inhalation or ingestion. Those formulating or handling BPA directly have the highest inhalation risk. Studies have shown that the release of BPA from products like epoxy liners is dependent upon temperature. Heat and age can both increase BPA migration.
The result of BPA exposure has been actively debated. The National Toxicology Program, an office within the National Institutes of Health, acknowledged in a draft report that the chemical might cause cancer and other serious disorders. However, further studies conducted by the U.S. National Toxicology Program have shown that there is insufficient evidence to state that BPA is carcinogenic or mutagenic. Groups directly related to the manufacture and sale of BPA claim that current exposure levels are well below permissible limits. They also cite reports that refute the claims of the CERHR. Studies by the European Union show that BPA exposure at “realistic” doses does not have any effect on reproduction or development. With the debate over the safety of BPA ongoing, it would be wise to carefully examine applications involving direct contact with food and drink or medical devices.

Table 1. BPA Concentration in bisGMA from Various Manufacturers
How Should Manufacturers Respond?
So how should a formulator/manufacturer of BPA-containing products respond to these reported hazards? First, a manufacturer should know how much BPA is incorporated into their final products, whether directly or indirectly. This means choosing raw-material suppliers who are forthcoming with their BPA specifications. For example, bisGMA is a commonly used resin in dental adhesives and sealants. With Esstech-supplied bisGMA, average BPA content can be decreased from 180 ppm to 2 ppm (see Table 1). In addition, Esstech also supplies pre-mixed dilutions of bisGMA with triethylene glycol dimethacrylate (TEGDMA).
Table 2. BPA Concentrations in Various Dimethacrylate Resins

Figure 2. PMDM

Figure 3. Sodium Salt of NTG-GMA

Figure 4. PMGDM (Para Isomer)
Glass ionomer cements (GIC) are common BPA-free adhesives used in the dental industry. These cements are two-component systems comprised of a mixture of polymerized acids and specifically designed glass powder. When mixed, the acids begin to partially dissolve the glass particles. The acid polymers then combine chemically with the dissolved glass components to produce a hard matrix material. The final matrix is reasonably translucent, allowing for various formulator manipulations to occur. GICs work well in moist environments, and are good for bonding ceramics to metals. In the dental industry, GICs are also used to bond gold and base metals to resin composites. Their utility allows them to be applied directly to clean substrates without additional preparation.
Research and development has focused on creating resin-modified glass ionomer cements. Resin-modified GICs are still considered BPA-free and add an additional method of curing. Chemists have created an adhesive system with increased toughness that can be chemically cured or photo-cured by introducing resins to GICs.
The ongoing debate over the risks associated with BPA can make it difficult to set a clear course of action. Those handling BPA directly or indirectly should ask themselves the following questions.
- Does my product create a BPA inhalation or ingestion risk?
- Is BPA a crucial component of my system?
- Is there an alternative supplier available whose material is lower in BPA?
- Can the BPA-containing material be replaced with a BPA-free alternative?
About the Company
Esstech Inc. develops and manufactures advanced materials for the dental, optical, cosmetic, and coating industries. With chemistries that meet medical purity standards, customers worldwide rely on Esstech for technical resins, polymerization initiators, adhesion promoters, surface modifiers, purified monomers and glass nano-fillers.For more information, contact Esstech Inc., 48 Powhattan Ave., P.O. Box 39, Essington, PA 19029; phone (610) 521-3800 or (800) 245-3800; fax (610) 521-4600; e-mail techsupport@esstechinc.com; or visit www.esstechinc.com.
Disclaimer
The claims made in this article are believed to be accurate, but all are made without guarantee since the conditions of use are beyond Esstech Inc.’s control. The information is solely provided as a guide. Esstech Inc. disclaims any liability in connection with the use of the information, and does not warrant against infringement by reason of the use of its products in combination with other materials or processes.Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








