
Figure
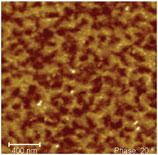
1. Film structure seen in an AFM picture of a mono-modal dispersion. Tapping
mode: dark areas = soft, bright = hard phases
Acrylic polymers for pressure-sensitive adhesives (PSA) have been known to researchers for a long time. Polyacrylates with a specific monomer composition can be used as PSA without additional compounding or formulation. The application properties of polyacrylates adhesives are, therefore, mainly achieved through the polymer properties. More accurately, the film properties of the polymer determine the application properties of such adhesives.
Polyacrylates can be synthesized through solution- and emulsion polymerization, and can be applied as dispersion, a solution or as a reactive hot melt. Films that are formed from solution and reactive hot melts are mainly smooth and structure free, whereas polymer films formed from dispersions have well-defined structures (see Figure 1). The figure shows an AFM picture of a mono-modal dispersion after drying for 3 minutes at 90°C and then being cryo cut. The boundary areas of the former polymer particles are clearly seen, even though the film was completely dried. The question arises whether these structures that are found influence our adhesive properties and, if so, can we control these structures or application properties?

Figure 2. An illustration of the inverse proportionality that exists between peel and shear.
The aim of this research was to obtain specific polymer particle structures in waterborne acrylics that provide structured films with high performance application properties, increasing both shear and peel strength.
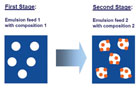
Figure 3. Two-stage emulsion polymerization to create structured polymer particles.
Structured Particles
Methods for creating structured particle morphologies in emulsion polymerization have been known for several years.1Usually, a two-stage polymerization process is performed. In the first stage, an emulsion with a certain composition is fed into the reactor, followed by the second stage with another composition. Depending on the thermodynamic equilibrium as well as the kinetics of the polymerization, different particle morphologies can be obtained. As shown in Figure 3, a swelling polymerization process can lead to heterogeneous structures of varying size. Here, small domains depending on the thermodynamic and kinetic factors are obtained; for example, Figure 3 shows a “raspberry” structure. During film formation, the structured polymer particles form structured films. The morphology of these films may or may not resemble the particle morphology, thus resulting in different application properties.
Figure 4. Tensile strength of three different polymers, each made of 75% BA and 25% MMA (white line shows two-stage polymer, yellow line shows one-stage copolymer, and orange line is blend of 75%PBA and 25%PMMA).
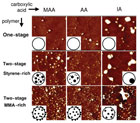
Figure 5. Cross-sections of dried dispersions with different chemical composition and morphology [TM-AFM phase images, 2µm × 2µm]. Different columns illustrate dispersions with different carboxylic acids, whereas the changes implied by swelling polymerization are shown in different rows. The insets are used to schematize the observed particle morphology.
Controlling the Morphology of Structured Particles
As mentioned earlier, the morphology of dispersion particles can be controlled thermodynamically or kinetically.2In a fundamental study, the effect of the acid type (acrylic acid, methacrylic acid and itaconic acid) on dispersion particles with different morphologies (no morphology, styrene microphases and methyl methacrylate microphases) was investigated. The different particle morphologies as seen through an atomic force microscope are seen in Figure 5. Figure 6 shows the results of shear and peel testing of the dispersions, formulated with 25% of tackifier resin.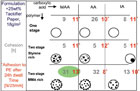
Figure 6. Shear and peel (*) of dried dispersions with different chemical compositions and morphologies, as was shown in Figure 6.
These results further emphasize the importance of micro-phase separation in polymer films to enhance application properties.
Figure
7. 2-D structure of a binary polymer solution
after spinodal de-mixing showing bi-continuous phases.
Polymer Films with Structures Resembling Bi-Continuous Structures
The question that arises is whether it would be possible to create bi-continuous polymer films from structured polymer particles. While the analysis of bi-continuous structures in films is difficult, our aim was to find a phase-separated polymer film that resembled a 2D-structure of a binary polymer solution after spinodal de-mixing (see Figure 7).
Figure 8. Cross-sections of dried dispersion films (Dispersion A, Dispersion B, a mixture of A and B, as well as a two-stage polymer with the composition of A and B) measured by AFM [TM-AFM phase images, 2µm × 2µm].
In the AFM pictures of the original dispersions, the boundary structures mentioned before are clearly seen. This is also the case in the mixture, although the film is phase-separated. A totally different picture is seen for the film of the two-stage polymer. Here a microphase-separated film is seen with no structures between the polymer particles. In fact, one is not even able to distinguish the different particles, which were 200 nm large.

Figure 9. Comparison of the adhesive properties of a mixture of Dispersion A and Dispersion B and the stage polymer with the composition of A and B.
Summary
A tailored particle morphology achieved by a staged-emulsion polymerization process provides special polymer film morphologies. Either small-sized polymer domains or bi-continuous film structures provide premium adhesive properties, which cannot be obtained by a blending process. Advanced polymer design, well-fitting formulation know-how and the newest coating technology continuously result in new classes of high-performance adhesives. And we are happy to provide all of this to our customers in order to make them more successful.Acknowledgements
Michael Kutschera, Ph.D., BASF-AG, Ludwigshafen, GermanyNok-Young Choi, Ph.D., BASF-AG, Ludwigshafen, Germany
