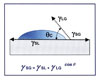
This phenomenon, and the concept of contact angle measurement as a means to describe it, is best defined by Young’s Equation (see Figure 1). Simply stated, the phenomenon is the interaction between the forces of cohesion and the forces of adhesion, which determines whether or not a liquid will spread over a surface or “wet it out.”
Surface tension is not simply a property of the liquid alone; it is also a property of the liquid interface between that liquid and other surrounding mediums (the gas and the solid). Based on the materials being studied, this relationship is what causes a portion of liquid to be attracted to another solid surface, such as film, paper or release-coated liner. Surface tension has the dimension of force per unit length or energy per unit area. Surface tension and surface energy are related, but we typically use the term surface energy when referring to energy per unit of area. This is a more specific term, in the sense that it applies to solids.

Surface Energy and Wetting
Surface energy and wetting are important material considerations in a variety of industries, including coatings, packaging, printing and converting, and pressure-sensitive adhesive materials manufacturing. In manufacturing, printing and converting operations, we need to know if the surface we are attempting to coat or print will readily accept the applied coating. If we are using a water-based formulation, we need to know if the substrate is hydrophilic (i.e., it likes water or some other liquid) or hydrophobic (i.e., it repels water or some other liquid). This will give us an indication of how well the adhesive or ink liquid will spread out, or wet, over the surface (see Figure 2).


Surface Energy Measurement
Two common methods are used for measuring surface energy: dyne measurement and contact angle measurement.Dyne Measurement
Of the two methods, the dyne measurement method (which uses a simple solution applied to the solid substrate) is the easier, faster and less costly option. This method is based on ASTM D 2578. The most common solution used comprises ethyl cellosolve, formamide and a dye that makes the solution easier to detect with the naked eye. The variation in concentrations of the ethyl cellosolve or the formamide results in different dyne level solutions.
In this process, dyne solutions of various dyne level concentrations are applied to the substrate until one is found to completely wet-out the surface. The dyne level of the substrate then corresponds to the dyne level of that solution. Three solution application processes are available: cotton swab, dyne pen, and a draw-down coating using a lab draw-down coater. Variations in the pressure applied by the operator when applying the dyne solution, as well as the speed of the solution application, can affect the dyne result.
While the dyne solution method of surface energy measurement is faster, easier and less costly, it also suffers from the inherent problems of being subjective and inconsistent. The dyne method may be less effective because it:
- Is open to potential contamination of the pens or applicators, which can affect results
- Requires the mechanical spreading of the dyne solution over the surface of the substrate in order to allow the solution to reach equilibrium, which takes an imprecise time period
- Does not allow for an easy true average measurement of the surface energy of the substrate
- Requires the user to “read” and interpret the behavior of the dyne solution on the substrate
- Typically results in variations of 3-10 dynes/cm in measurement of the same surface
It should also be noted that dyne solutions have a limited shelf life of only about six months; the use of outdated dyne solutions can result in false dyne level information. Dyne testing is best-suited to shop floor/production testing that is used as a quick check of previously established information, such as that recorded after substrate treatment.

The surface energy of a solid substrate such as a release-coated liner or a label facestock cannot be measured directly, because solids do not change shape in reaction to their surface energy. Because of this, the use of contact angle measurement is the most reliable method to measure the surface energy of such solids. Practical measurements of surface energy use the interaction of a liquid droplet of a test solution resting on the solid surface.
The term contact angle, which actually describes the shape of a liquid droplet resting on the solid surface, comprises a tangent line from the liquid droplet to the solid surface. The higher the surface energy of the solid substrate in relation to the surface tension of the liquid, the smaller the resulting contact angle.
Most instruments used for measuring contact angles are based on the observation and measurement of the tangent line of the liquid drop placed on the solid substrate surface. Contact angle measurement instruments fall into three general categories:
- Video-based, with a computerized automated contact angle calculation system
- Microscope-based, with an ocular viewing tube and internal protractor
- Projection screen-based, with an amplified image and protractor screen
The video-based system is quite accurate and-because it includes a CCD camera-offers the advantage of being able to maintain visual records of completed contact angle measurements. However, video-based systems are quite costly. Microscope-based systems are low power and less accurate because they rely to a greater extent on operator interface and decision making.
Projection screen-based systems are relatively simple to use and offer the added benefits of ease of droplet formation and location, as well as ease of operator viewing of the test in progress. In most types of projection screen-based systems, it can be difficult for the operator to properly identify the tangent line and the contact point of the liquid/solid interface.
All contact angle meters function in essentially the same manner to determine the contact angle, and they require some level of operator experience in order to determine the exact interface point of the liquid drop with the solid surface. This point is needed to properly determine the correct contact angle.
However, a much easier and more reliable method employs a concept called the half-angle measurement technique (U.S. Patent No. 5,268,733). The half-angle measurement technique is available on the CAM-PLUS series of contact angle meters (see Figure 3). This technique enables the operator to easily identify the liquid/solid interface and the apex of the liquid drop that is used in calculating a half-angle. The half-angle can be determined by following a series of steps:

2. Place the solid substrate sample onto the specimen holder.
3. Locate the sample to be tested precisely under the syringe tip.
4. Release one drop (10 screen divisions wide) of the water solution from the syringe.
5. Move the syringe and raise the sample surface to contact the drop.
6. Adjust the height of the sample surface to align it with the display horizontal cross-line.
7. Align the left side of the drop with the display vertical cross-line.
8. Locate the apex (centerline) of the drop.
9. Align the protractor hairline indicator with the apex of the drop.
10. Read the half-angle on the protractor scale.
A diagram of the half-angle technique is shown in Figure 4. The contact angle (u) is then calculated using the formula:
contact angle u = 2X arc tan (drop height/drop radius)

The half-angle technique has substantiated measurement-to-measurement repeatability of ± 1 dyne/cm for tests completed by the same operator. The technique also offers excellent repeatability of ± 2 dynes/cm for tests completed between different operators or different locations.
As previously mentioned, one of the drawbacks of using dyne solutions or dyne pens is the significant variation (3-10 dynes) that one can expect between measurements of the same substrate surface by the same operator. The half-angle technique for determining contact angle is therefore 1.5 to 5 times more accurate than using dyne solutions or dyne pens. Thus, the half-angle technique offers repeatability that is far superior to the use of dyne solutions or even other contact angle measurement techniques. Examples of high and low contact angles using the half-angle technique are shown in Figure 5.
Summary
The acceptable wet-out of adhesives on solid surfaces, or inks on printing surfaces, is essential to the production of suitably performing products. The characterization of adhesive, ink or coating wettability of solid materials has presented challenges to both scientists and printing and coating technicians alike.For converters and printers of plastic films, it is important to know the surface energy of the solid surface and the surface tension of the liquid that will be applied to it. The proper balance of liquid component surface tension and solid component surface energy is critical to ensuring good coating adhesion and acceptable print quality.
Dyne testing of the solid surface is a good basic test methodology; contact angle measurement is a better, more consistent process; but the half-angle measurement technique may be the most accurate and most repeatable measurement option of all.
For more information, contact Chemsultants International, 9079 Tyler Blvd., Mentor, OH 44060; (440) 974-3080; fax (440) 974-3081; e-mail info@chemsultants.com; or visit www.chemsultants.com.
References
1. Förch, Renate, Schönherr, Holger, Tobias, A., Jenkins, A., Surface Design: Applications in Bioscience and Nanotechnology, Wiley-VCH, p. 471, ISBN 3527407898, 2009.2. de Gennes, P.G., “Wetting: Statics and Dynamics,” Reviews of Modern Physics, pp. 827-863, 1985.
3. Tadmor, Rafael, “Line Energy and the Relation Between Advancing, Receding, and Young Contact Angles,” Langmuir, 20: 7659, 2004.
4. Israelvilli, Jacob, Intermolecular and Surface Forces, Academic Press, 1985-2004.
5. Van Krevelen, D.W., Properties of Polymers, 2nd revised edition, Elsevier Scientific Publishing Co., Amsterdam-Oxford-New York, 1976.
6. Shieh, Sarah, An Analysis of Contact Angle Measurement, AST Products, March 2001.
7. Blitshteyn, Mark and Wetterman, Bob, Testing for Surface Energy, Converting Magazine, Delta Publications, 1993.
8. Wetterman, Robert P., “Surface Tension Measurement and Coatings Development,” Paint & Coatings Industry, pp. 202-206, October 1998.
9. ASTM D2578 (Test Method for Wetting Tension of Polyethylene and Polypropylene), and ASTM D5946 (Test Method for Corona Treated Polymer Films using Water Contact Angle Measurements), ASTM International, 100 Barr Harbor Dr., P.O. Box C700, West Conshohocken, PA, 19428-2959 USA, 2010.
10. TAPPI T-458 (Surface Wettability of Paper-Angle of Contact Method), TAPPI, 15 Technology Parkway S., Norcross, GA, 30092, 2010.
11. Utschig, Steven, Measuring Treatment of Non-Porous Materials, Enercon Industries Corp., 12/22/2006.