
Hot-melt adhesives based on metallocene polyolefin copolymers, such as AFFINITY™ GA Polyolefin Plastomers, or "POPs," available from The Dow Chemical Co., have demonstrated significant advantages over traditional EVA-based HMAs. These new-generation HMAs provide end users with improvements in processability, performance, cosmetic appearance and total cost.
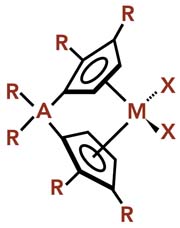
M is a transition metal, usually group 4b (Zr, Ti, Hf); A is an optional bridging atom, generally Si or C atom with R=CH3; R=H, alkyl, or other hydrocarbon groups, which need not all be identical; X=halogen atom (generally CI) or alkyl group.
Business Needs Make New HMA Technology Essential
Why are these advantages important? Today the world runs faster and harder than ever before. From packaging lines to diaper manufacturing, everyone is looking for a more efficient way to produce and package products and materials, increase line speed, reduce down time, and lower energy and maintenance costs. What's more, the adhesives industry is constantly looking for more versatile adhesives that work on a variety of substrates and in the widest possible range of service temperatures. Hot-melt adhesives formulated using novel metallocene polyolefin catalyst technology meet and exceed these needs.But before we take a closer look at the performance and economic advantages of metallocene catalyst-based HMAs, a discussion of the chemistry behind these important polymers is needed.
A catalyst is a material that modulates a chemical reaction; it can increase the reaction speed or the efficiency, or - on a molecular level - determine which molecules react together and how they form a molecular structure. The origins of metallocene catalyst technology can be traced to the 1950s, when Italian1 and American2 scientists discovered that ethylene could be homo polymerized in the presence of titanocene dichloride and aluminum alkyls. Later, research by Ewen3 and Kaminsky4 increased the pace of development until, in the 1990s, the full industrial significance of these catalysts - and the versatility of new polymers that could be manufactured using them - was clearly understood.

Designing Molecules to Exhibit Specific Behavior
Metallocene catalyst technology allows the ability to design molecules based on their desired behavior. For example, an ethylene/octene copolymer can be designed with enough octene in the molecular structure to reach its theoretical amorphous density (0.855 g/cc). This represents a significant breakthrough for adhesive applications, because amorphous polymers provide better adhesion. While amorphous density is important, crystallinity is also needed to give the polymer added strength. However, higher crystallinity reduces adhesive properties. Metallocene catalyst technology allows the level of crystallinity in the polymer to be precisely controlled so that the optimum balance of adhesive properties and strength can be achieved.Precise control of properties also enables metallocene catalyst technology to produce polymers with specific molecular weights and narrower molecular weight distributions than competitive materials. This narrower molecular weight distribution is beneficial, as the targeted viscosity or molecular weight is produced without extraneous higher- and lower-molecular-weight components that generally detract from the desired product performance.
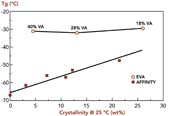
Because higher levels of octene are used to produce low-crystallinity polymers based on metallocene catalyst technology, the glass-transition temperatures of the polymers are lower than those of EVA grades used in HMA formulations (see Figure 3). Low glass-transition temperatures make the polymers more suitable for low-temperature HMA applications.

HMA Performance and Economic Advantages
The Dow Chemical Co. has been among the leaders in the development of metallocene catalyst and product-development research. In the early 1990s it developed the proprietary INSITE™ Technology and launched products based on this technology, including AFFINITY polyolefin plastomers, or POPs. In 2004, Dow introduced AFFINITY GA POPs for the HMA market, one of the most significant product introductions in the 35 years since the commercial introduction of EVA copolymers in the marketplace.5-6The physical properties of three AFFINITY polymers are shown in Table 1. AFFINITY GA 1900 and 1950 are intended for use in HMAs for case and carton sealing and graphic arts. AFFINITY EG 8200 is a low-melt-index polymer for use in diaper construction and graphic arts applications.
The performance and economic advantages of AFFINITY GA POPs have been well documented in the laboratory, as well as in actual use in HMA formulations. Hot-melt adhesives based on these metallocene polyolefin copolymers combine impressive performance with customer-demonstrated cost savings. They outperform EVA-based HMAs in processability, adhesive performance, cosmetic appearance and cost for end users. See Table 2 for more benefits.
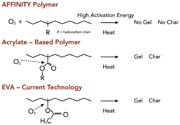
The odor, color, and workplace environmental advantages associated with these polymers are the result of a unique molecular structure. The AFFINITY GA polymer backbone is pure hydrocarbon, as shown in Figure 4. It does not contain any oxygen atoms or double bonds. This results in excellent thermal and viscosity stability (see Figure 5). When these polymers are combined with other pure hydrocarbon components in the formulation of HMAs, the result is excellent clarity and color stability (see Figure 6). Higher stability reduces charring, plugging, clogging and other oxidation-related application equipment problems, and results in very low or non-existent adhesive-related odor in the packaging workplace.

Cost Savings
High-flow HMA formulations based on metallocene polyolefin copolymers offer lower overall cost compared with EVA- or ethylene-n-butyl acrylate (EnBA)-based HMAs. In one case study, a pound of HMA-based on AFFINITY GA POPs sealed significantly more cases than a pound of a traditional polyolefin-based HMA. The mileage advantage in this case study resulted in reported savings of 15-30%. These savings were the result of aggressive bonding, improved thermal stability and lower density.
In another case study, a hot-melt adhesive line that had previously changed filters and nozzles on a monthly basis switched from an EVA-based HMA to a HMA based on an AFFINITY GA POP. The plant operated for a period of 18 consecutive months with no need to change nozzles and filters. This resulted in a tremendous decrease in downtime.
Tackifier Selection
The performance of a hot-melt adhesive is closely related to the compatibility of its components. The tackifier is a crucial formulation component, and studies were undertaken to assess the compatibility of metallocene catalyst polyolefin polymers with several types of tackifiers. The compatibility of a resin with a given polymer depends mainly on its polarity and, to a lesser extent, on its molecular weight. The high-molecular-weight fraction of the resin, measured by its Mz value, can lead to symptoms of incompatibility such as turbid melts or migration upon aging. Therefore, it is customary to measure the compatibility of a hot-melt adhesive by determining its cloud point. There are problems with this method, however; it is time consuming and the wax component can often obscure a polymer's cloud point.
Applications for Metallocene Polyolefin Copolymer HMAs
Metallocene polyolefin copolymers such as AFFINITY GA POPs are used today in hot-melt formulations for a variety of applications, including the following.- Case and carton sealing - including folding carton sealing, corrugated container closure, tray forming and pallet stabilization.
- General packaging - including food and beverage packaging.
- Bottle labeling - including roll feed and magazine feed applicators.
- Graphic arts - including lay-flat, hardcover and soft-cover applications.
- Multi-wall and specialty bags - including film laminating, pinch bottom, spot paste, valve assembly, longitudinal seam and bottom paste, plastic bags, vacuum bags, security bags, and wax bags.
- Woodworking - including adhesives for profile wrapping and edge banding.
- Nonwoven hygienics - including diaper construction adhesives.

Conclusion
Metallocene polyolefin copolymers are revolutionizing many markets, including adhesives. The benefits of being able to design a polymer to meet specific requirements have only just begun to be fully appreciated and used in the marketplace. As "designer" polymers continue to evolve to meet the needs of the market, many new and exciting polymers will emerge that will help grow current adhesives markets and possibly even create new ones.
For more information on metallocene polyolefin copolymers, contact the Dow Chemical Co., 2030 Dow Center, Midland, MI 48674; phone (800) 441-4369; or visit http://www.dowaffinityga.com or http://www.dow.com.
REFERENCES
1. Natta, G.; Pino, R.; Mazzanti, G.; Lanzo, R. Chim. Ind. (Milan), 39, 1032 (1957).2. Breslow, D.S.; Newburg, N.R. "Bis-(Cyclopentadienyl)-Titanium Dichloride-Alkylaluminum Complexes as Catalysts for the Polymerization of Ethylene," J. Am. Chem. Soc., 79, 5072 (1957).
3. Ewen, J.A.; Jones, R.L.; Razavi, A. "Syndiospecific Propylene Polymerizations with Group 4 Metallocenes," J. Am. Chem. Soc., 110, 6255 (1988).
4. Kaminsky, W.; Kulper, K.; Brintzinger, H.H.; Wild, F.R.W.P. "Polymerization of Propene and Butene with a Chiral Zirconocene and Methylalumoxane as Cocatalyst," Angew. Chem. Int. Engl. Ed., 24, 507 (1985).
5. Yalvac, S.; Karjala, T.P. "Novel High Flow Polymers and Their Applications," SPE ANTEC Proceedings, Chicago, p. 3453 (2004).
Yalvac, S.; Karjala, T.P.; Martin, L. "High Flow AFFINITY-Based Hot Melt Adhesives," ASC Fall Convention and Exhibition, Pittsburgh (2004).
