Formation and Determination of Aldehydes in Amine Catalysts
An accurate analytical method can ensure that no or low concentrations of aldehydes are present in catalyst samples.
Tertiary amine catalysts used for manufacturing polyurethanes and polyisocyanurates are known to degrade over time, producing products such as aldehydes that can directly contribute to emissions of organic compounds. To remain within the tolerable exposure limits, an analytical method with sufficient accuracy is needed to ensure that no or low concentrations of aldehydes are present in the catalyst samples.
Recently, a systematic analytical approach was reported for the accurate determination of aldehydes present in amine catalysts or amines by liquid chromatography using ultraviolet (UV) detection of their 2,4-dinitrophenylhydrazone derivatives.1 Based on various factors controlling the derivatization of aldehydes, optimum conditions were selected for the accurate determination of aldehydes. In addition, probable reaction mechanisms in both acidic and basic conditions were highlighted.
Since amine catalyst solutions in water are basic, it is important to have a clear understanding of the derivatization of aldehydes present in these materials, specifically, by looking at the effects of solution pH, derivatization reaction time and the number of active nitrogen atoms in amines. It was found that only a small fraction of aldehydes present in the amine can be derivatized in a basic environment. It appeared that most of the reaction steps in the near neutral and basic environments are kinetically slow and equilibrium limited. Ideally, the derivatization using 2,4-dinitrophenyl-hydrazine (DNPH) can be completed in an acidic environment, specifically, at pH around 2 with an excess amount of DNPH.
In this work, aldehyde data for four amine catalyst samples containing 1-3 nitrogen atoms have been obtained. The structures and chemical names of these amine catalyst samples are provided in Figure 1. This article intends to address issues related to the formation and accurate determination of aldehydes in amine catalysts used in the polyurethane industry.
Materials and Methods
The experimental protocols for the determination of aldehydes are similar to those detailed elsewhere.1 A Waters 2695 Alliance® system with a Waters 2998 diode-array detector was used. The system automation, data collection and data analysis were carried out with Waters Empower® 2 software. Experimental set up includes the following: column was 7.5 cm x 0.46 cm internal diameter, Kinetex® 2.6 µm, XB C-18, 100 Å column from Phenomenex®; injection volume was 10 µL; column temperature was 40°C; solvent flow rate was 0.5 mL/min under an isocratic condition of 40:60 (v/v) water-acetonitrile mixture; run time was 15 min.
The amine catalyst solution pH was controlled by adding 2N hydrochloric acid and was measured by a model S220 pH meter from Mettler-Toledo. The aldehyde concentrations were determined by high-performance liquid chromatography using a UV detector at 365 nm to detect aldehydes as their 2,4-dintrophenylhydrazone derivatives.
Reagents and Samples
Aldehyde standards as their 2,4-dinitrophenyl-hydrazones were obtained from Supelco. Liquid chromatography-grade acetonitrile was from Fisher Scientific, and water was Milli-Q grade. All amine catalysts are aged (or degraded) samples having much higher amounts of aldehydes than those determined in the fresh samples or in samples stored after blanketing with an inert gas such as nitrogen.2,3
Derivatization and Determination of Aldehydes
For standard determination of aldehydes, an amine sample solution pH was maintained at around 2 and the derivatization time was 20 min. For this, 2% catalyst sample was prepared with water where 2N hydrochloric acid was added to decrease the solution pH to about 2. 200 µL of this solution was transferred to a 1.5 mL chromatography sample vial. 1,000 µL of 0.06% (w/v) DNPH solution in acetonitrile was then added to the vial. The vial was capped and shaken well before placing in a 40°C heater block for 20 min.
For pH and heating time studies, 2% (w/w) amine catalyst solutions were prepared with water and 2N hydrochloric acid. The solution pH was varied by adding different amounts of acid. For derivatization, typically 200 µL of the above amine solution was transferred to a 1.5 mL chromatography sample vial. 1,000 µL of 0.06% (w/v) DNPH solution in acetonitrile was then added to the vial. The vial was capped and shaken well before placing in a 40°C heater block for different time intervals.
Oxidation of Amine Catalyst with Ambient Air
For ambient oxidative degradation at room temperature, 5 mL of each amine catalyst was transferred in a 20 mL bottle; the bottle was capped and left at room temperature. This provision allowed sufficient air in the liquid headspace for oxidation. Periodically, a portion (0.2 g) of each sample was withdrawn to determine the concentrations of aldehydes formed in the respective aliquot.
Degradation of Tertiary Amine Catalyst and Formation of Aldehydes
Oxidation of tertiary amines has been studied extensively.2, 4-13 Earlier studies suggested that oxygen uptake by tertiary amines requires the presence of an oxidation product from catechol, presumably free radicals4 and an amine can be broken down to produce free radicals by irradiation.5 Recently, it was reported that formation of amine radical cations by visible light can be facilitated by photoredox catalysts.13
Aldehydes and ketones were obtained from amines containing hydrogen attached to a carbon ato nitrogen when they were oxidatively hydrolyzed by buffered permanganate in warm aqueous t-butyl alcohol.6 Oxidation of amines was carried out at both ambient7 and elevated oxygen pressure.9 The relatively slow autoxidation at ambient oxygen pressure was hypothesized to be dominated by a-CH abstraction. An initial electron transfer can form an amminium radical cation, followed by loss of a proton to form a-CH radical that can react with oxygen to form the a-hydroperoxy compound.7, 9
The rate of autoxidation was found to be pH dependent; below pH 6, it is very slow but increases considerably at higher pH, reaching a maximum at pH 9.7 Similar aldehyde formation was also postulated through electrochemical oxidation of tertiary amine due to formation of a radical cation, followed by its deprotonation to give a radical that degrades further to provide an aldehyde as a product.10 At elevated oxygen concentration (in excess of 20 bars of oxygen), a tertiary amine yields amine oxide as a major product.9 However, amine oxide was not found to be an intermediate for the formation of formaldehyde.8
Oxidation of tertiary amines such as N,N-dimethylaniline produced formaldehyde as a result of demethylation carried out by a reaction with hydrogen peroxide or hydroperoxide in the presence of a catalyst such as horseradish peroxidase.8 Reactions of primary aliphatic amines11 and tertiary amines12 with nitrate radicals were studied. In the case of a tertiary amine, the nitrate radical was found to abstract hydrogen atom from one of the alkyl groups resulting in an amine alkyl radical. The latter reacts with an oxygen molecule to form an aminyl alkylperoxy radical that was reduced to an aminyl alkoxy radical (in this case, by reacting with NO).12 Based on these observations, possible degradation mechanisms for the formation of formaldehyde from a tertiary amine catalyst are provided in Figure 2.
Figure 3 provides examples for the formation of formaldehyde from Catalyst 1 and Catalyst 3 with time under ambient oxygen pressure. Acetaldehyde concentrations are also plotted. In both cases, the initial acetaldehyde concentrations are found to remain constant during the time period. This suggests that acetaldehyde is not a product from oxidative degradation of these amine catalysts, as predicted from Figure 2 with R1 and R2 being –CH3.
It appears that Catalyst 1, which contains an –OH group in the b-position, had a faster rate of formaldehyde formation than Catalyst 3 with just a –CH3 group attached to the N atom. This is consistent with a previous observation that an –OH group in the b-position results in a faster rate of oxidation and in the g-position displays a reduced rate of oxidation.7 These observations justify the necessity for storing amine catalysts in an oxygen-free environment.3
Effects of pH and Reaction Time on DNPH Derivatization
Effects of pH were examined by derivatizing samples with DNPH at several pHs and different heating (or reaction) times and, subsequently, determining aldehyde concentrations by liquid chromatography. Here, the derivatization temperature was set at 40ºC. The results pointed to an optimum pH of about 2.0 and a reaction time of 20 min for complete derivatization of aldehydes in an amine catalyst.1
An example for the variation of formaldehyde concentrations in Catalyst 2 at two solution pHs of 2.1 and 8.7 with heating time is provided in Figure 4. It is observed that formaldehyde concentrations reach a constant level after about 10 min at pH 2.1. However, at pH 8.7, the formaldehyde concentration continues to increase with heating time, and the values are much lower than the constant value reached at pH 2.1. Similar results for Catalyst 4 can be found in our earlier work.1
Another approach to explore the pH dependence of derivatizing aldehydes can be to vary the pH of the 2% amine solution by adding 2N hydrochloric acid (HCl) in incremental amounts prior to carrying out the derivatization. With this, aldehyde data were obtained at pH values ranging from 1.0 to 11.8. Figure 5 shows plots of formaldehyde concentrations for four catalyst samples against the volume of 2N HCl. HCl concentration of 0 is for derivatization carried out with 2% amine solutions in water (without acid). Other data are for derivatized samples prepared with water and various amounts of 2N HCl.
The pH of each solution after thorough mixing was measured and plotted in Figure 5. For all amine samples, the overlaid plots of pH and aldehyde concentration in ppm against the volume of HCl in µL show sharp transitions in the concentrations of aldehydes at around pH 7 where the amine is completely neutralized. Beyond these transition points, the solutions become acidic with pH ≤ 2.2. The transition points vary in the volume of 2N HCl. Catalyst 1 shows a transition point between 1,000-1,100 µL of HCl. Other transition points lie between 1,100-1,200 µL of HCl for Catalyst 2, between 1,200-1,300 µL of HCl for Catalyst 3, and between 1,400-1,500 µL for Catalyst 4.
It appears that amine equivalent weights dictate where the equivalent points should be. Aldehydes are known to form N-substituted hemiaminals14 that are expected to dissociate after neutralization with acid. Assuming that all nitrogen atoms are protonated with an equivalent amount of hydrochloric acid, the number of nitrogen atoms n should be important in determining the volume of acid. When x µL of hydrochloric acid of known normality N are added to be a part of the total volume V mL, the molar acid concentration is given by N x /(1,000 V). Similarly, when y grams of amine having molecular weight M are added to total volume V mL, the molar amount of amine is 1,000 y/ MV and the equivalent amount is 1,000 y n/ MV.
We note that molecular weights are 190.0, 89.1, 160.3, and 203.0 g/mol, for Catalysts 1, 2, 3, and 4, respectively. From these, the volume of hydrochloric acid x needed to reach the neutralization point is obtained by the following equation:1
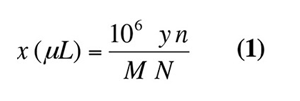
With V=10 mL, y = 0.2 g, and N=2, we can calculate the values of x as listed in Table 1. The calculated neutralization points are in excellent agreement with those observed in Figure 5. However, a slight excess of acid (such as 100-200 µL of 2N HCl) relative to the neutralization amount is recommended to ensure that the derivatization of aldehydes is carried out in an acidic environment.
Mechanisms of Derivatization
It is clear that aldehydes can be derivatized by DNPH in both acidic and basic environments. However, the difference lies in the extent to which the aldehydes are converted to their corresponding hydrazones to be determined by liquid chromatography. Obviously, the mechanisms of hydrazone formation in the acidic and basic media are different, as detailed in a recent work.1 It was proposed that hydrazone formation in acidic environment involves two major steps. The first step is protonation of the carbonyl group followed by nucleophilic attack of the amino group from DNPH to form a carbon–nitrogen single bond. In this case, nucleophilic attack of the carbonyl carbon by hydrazine is catalyzed by acid (H3O+), which protonates the carbonyl oxygen and after rearrangement the positive charge shifts to carbonyl carbon (> +C−O−H). The second step involves proton transfer to form carbinolamine, and dehydration of carbinolamine to produce the hydrazone.
In a basic environment, a proton is not available to bind with the carbonyl oxygen, but the addition of hydrazine to a carbonyl carbon is still possible due to the dipole moment of the carbonyl (> C=O) bond. The greater electronegativity of the carbonyl oxygen results in a partial positive charge on the carbon atom, which makes the carbon atom susceptible to nucleophilic attack to form a carbinolamine zwitterion. Then the oxygen atom quickly abstracts a proton to form a neutral carbinolamine intermediate. The dehydration of carbinolamine proceeds through nitrogen deprotonation with an OH− group to generate a carbinolhydrazyl anion that rearranges with a loss of OH– to form the hydrazone. However, the hydroxide ion can also remove a proton from oxygen of the carbinolamine and regenerate the starting aldehyde or ketone and hydrazine.
It appears that two major reasons for the slow rate of reaction in basic environment are slower attack by DNPH on carbon atom of > C=O vs. > +C−O−H, and −OH is a poor leaving group. Because of several slow reaction steps and the last reversal step, DNPH derivatization in basic environment is expected to remain incomplete.
Conclusion
Amine catalysts can easily be subject to oxidative degradation. An experiment for autoxidation of two amine catalyst samples provides evidence that they can have additional formaldehyde when they are exposed to air even at ambient temperature. In these samples, acetaldehyde concentrations were found to remain unchanged from their initial values.
This work provides a rigorous approach to obtain the optimum conditions for determining aldehydes in amines through derivatization with 2,4-dinitrophenylhydrazine. In this regard, the understanding of reaction mechanisms is important to carry out an accurate determination of carbonyl compounds in amine catalysts. Results show that the formation of hydrazones from aldehydes present in an amine catalyst can occur in both acidic and basic environments. However, derivatization proceeds slowly and remains incomplete in near neutral and basic media. This can result in significant underestimation of aldehydes or ketones.
It is important to protonate all nitrogen atoms in the amine molecule. Thus, an acidic medium is needed for accurate determination of aldehydes using DNPH as the derivatizing agent. Aldehydes in an amine catalyst can be completely derivatized at around pH 2 with DNPH at 40°C using a 20-min heating period. Further, a simple quantitative calculation method is provided to neutralize an amine catalyst with hydrochloric acid and ensure an acidic environment for the DNPH derivatization.
The above analytical approach has been found to be useful not only for accurate determination of aldehydes in the amine catalysts, but also for developing appropriate additives to prevent oxidation,2 and to reduce aldehyde content in an amine catalyst.
For more information, contact the author at (281) 719-7604 or bhajendra_barman@huntsman.com, or visit www.huntsman.com.
Acknowledgments
This work was presented at the 2014 CPI meeting in Dallas. Many helpful discussions with Nadata Green, Donald H. Champion, Robert A. Grigsby, Jr., Matt Meredith, Gene Wiltz and Frank Rodriguez are gratefully acknowledged.
Disclaimer
All information contained herein is provided “as is” without any warranties, express or implied, and under no circumstances shall the author or Huntsman be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term “Huntsman” is used herein for convenience only, and refers to Huntsman Corp., its direct and indirect affiliates, and their employees, officers, and directors.
References
1. Barman, B.N., “Accurate determination of aldehydes in amine catalysts or amines by 2,4-dinitrophenylhydrazine derivatization,” J. Chromatogr.A, 2014, 1327: 19-26.
2. Barman, B.N., Grigsby, Jr., R.A., “Inhibition of Amine Oxidation,” U.S. Patent Publication No. 2012/0271026 A1, 2012.
3. Huntsman Technical Bulletin: Storage and Handling of JEFFCAT® Catalysts, 2005.
4. Beevers, H., James, W.O., “The Behavior of Secondary and Tertiary Amines in the Presence of Catechol and Belladonna Catechol Oxidase,” Biochem. J., 1948, 43: 636-639.
5. Hadley, S.G., Volman, D.H., “Photochemical Formation of Free Radicals from Aliphatic Amines as Studied by Electron Spin Resonance,” J. Am. Chem. Soc., 1967, 89: 1053-1055.
6. Rawalay, S.S., Shechter, H., J. Org. Chem., 1967, 32: 3129-3131.
7. Beckwith, A.L.J., Eichinger, P. H., Mooney, B.A., Prager, R.F., Aust. J. Chem., 1983, 36: 719-739.
8. Kedderis, G.L., Hollenberg, P.F., “Characterization of the N-Demethylation Reactions Catalyzed by Horseradish Peroxidase,” J. Biol. Chem., 1983, 258: 8129-8138.
9. Correa, P.E., Hardy, G., Riley, D.P., “Selective Autoxidation of Electron-Rich Substrates under Elevated Oxygen Pressures,” J. Org. Chem., 1988, 53: 1695-1702.
10. Adenier, A., Chehimi, M.M., Gallardo, I., Pinson, J., Vila, N., “Electrochemical Oxidation of Aliphatic Amines and Their Attachment to Carbon and Metal Surfaces,” Langmuir, 2004, 20: 8243-8253.
11. Malloy, Q.G.J., Qi, L., Warren, B., Cocker III, D.R., Erupe, M.E., Silva, P.J., “Secondary Organic Aerosol Formation from Primary Aliphatic Amines with NO3 Radical,” Atmos. Chem. Phys. Discuss., 2008, 8: 12695-12720.
12. Erupe, M.E., Price, D.J., Silva, P.J., Malloy, Q.G.J., Qi, L., Warren, B., Cocker III, D.R., “Secondary Organic Aerosol Formation from Reaction of Tertiary Amines with Nitrate Radical,” Atmos. Chem. Phys. Discuss., 2008, 8: 16585-16608.
13. Hu, J., Wang, J., Nguyen, T.H., Zheng, N., “The Chemistry of Amine Radical Cations Produced by Visible Light Photoredox Catalysis,” Beilstein J. Org. Chem., 2013, 9:1977-2001.
14. March, J., Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 4th edition, New York, Wiley, 1991, Chapter 16.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





