Over the last 50 years, the aerospace and aircraft industries have used silicone in an ever-growing number of applications. Silicone inherently has high dielectric strength (typically ≥ 500 V/mil) and a large coefficient of thermal expansion (CTE), allowing it to absorb stresses during thermal cycling. In addition, a low modulus and low glass-transition point enable silicone to remain elastic at low temperatures and resist breakdown at high temperatures or in ultraviolet (UV) light.
In addition to these characteristics, key to the legacy of silicone in an expanding range of applications is the material’s ability to be designed as needed to fulfill a particular role. New innovations in silicone technology that add to silicone’s prolific resume and success in the aerospace and aircraft industries include fuel- and solvent-resistant silicone elastomers, silicone ice-release coatings for aerodynamic surfaces, and controlled volatility silicone film adhesives.
Silicone Chemistry
Silicone owes its versatility to its chemistry. Depending on an application’s requirements, a silicone can be tailored for specific needs by filler incorporation and/or polymer alterations.
Silicone is comprised of repeating siloxane units (Si-O), with substituent groups attached to the open valences of the silicon atom. Having no carbon in the backbone, these repeating siloxane units are often referred to as a polysiloxane polymer, or a polyorganosiloxane polymer in reference to the organic substituent groups.
Typical substituent groups include methyl, phenyl and trifluoropropyl, which interact with the Si-O-Si units to cause the resulting silicone material to exhibit certain attributes. Conventional silicones, which are comprised of methyl groups, are commonly referred to as PDMS silicones because of their polydimethylsiloxane polymers. Trifluoropropyl (fluoro) or phenyl groups are often added in varying degrees to form trifluoropropyl methyl polymers or diphenyldimethylpolysiloxane polymers, respectively. Phenyl influences refractive index, permeability and operating temperature range. Fluoro-containing silicone products, also known as fluorosilicones, are often used for applications involving solvents and the need for swell resistance.
A silicone polymer is manufactured in several steps. Initially, a silicone polymer is produced via ring-opening polymerization (ROP). The process begins with polyorganosiloxane cyclics reacting with a chain-terminating species, or “end blockers,” in the presence of an acid or base initiator. The product of this polymerization reaction is a mixture of various molecular weight molecules, including cyclical and linear polymers of varying lengths; the concentrations of each species are based on a thermodynamic equilibrium. Because the uncrosslinked, lower-molecular-weight species are often volatile and will migrate out of the cured matrix over time, resulting in contaminants, it is necessary to remove low-molecular-weight polymers and cyclics from the polymer mixture through a refinement process.
For applications that demand minimal outgassing, controlled volatility (CV) silicones may be ideal. Primarily intended for applications that exist in a vacuum, CV materials are processed to be tested per ASTM E 595 and to meet specifications outlined in NASA SP-R-0022A and ESA PSS-014-702, which recommend a maximum allowable total mass loss (TML) of less than 1% and a collected volatile condensable material (CVCM) of less than 0.1%.1-3
With these materials, physical properties remain the same, but volatiles are minimized. Mitigation of volatiles in space and many terrestrial applications is essential because a vacuum environment compounds the propensity of volatiles to condense on or around device components at elevated temperatures. For example, the collection of volatiles within a solar cell’s light path can cut off equipment’s power generation. By greatly reducing outgassing, controlled-volatility silicone adhesives and encapsulants significantly aid in the protection of equipment from debilitations to performance.
Silicone Film Adhesives
Advances in silicone technology have led to the development of controlled-volatility, or low outgassing, silicone film adhesives. This is exactly what it sounds like: a tape-like silicone adhesive in film form. An alternative to (and arguably an improvement upon) the more traditional liquid silicone adhesive, the silicone film adhesive can be used in wire staking applications or to bond optical solar reflectors (OSRs) or solar cells, as well as to adhere appliqués to substrates.
The film adhesive is a two-part curable silicone with an adjustable cure time that at the longest runs approximately 24 hours at room temperature. The Part B of the film adhesive is a liquid activator applied like a primer, and Part A is a calendered sheet of uncured silicone that begins to cure on contact with the activator. The properties of a two-part film adhesive can be adjusted at the manufacturer’s level by adding fillers, such as carbon for static dissipation, to the Part A component.
In adhesion strength, the film adhesive performs similarly to liquid silicone adhesives—and with greater processing efficiency. A peel-and-stick application replaces mixing and de-airing for easier processing, faster turnaround times and controlled bond line thickness.
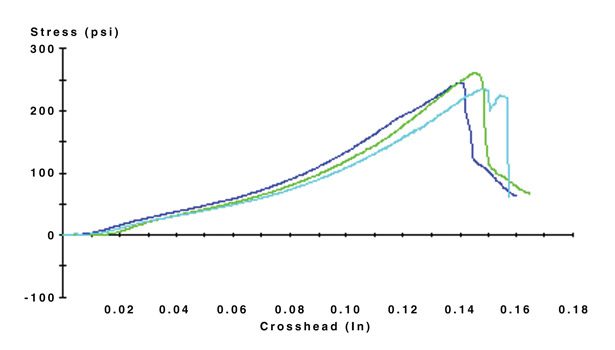
A lap shear test is typically conducted to quantify the adhesion strengths of silicone film adhesives. Per lot in three replicates, the films are bonded at 0.012 in., cured at 65°C, and pulled apart at 0.02 cm/min using an MTS loading frame. Figure 1 depicts the results for a two-part, unfilled silicone film adhesive tested for lap shear via these parameters.
In form, the silicone film adhesive is a far cry from the liquid silicone adhesive, but the two are very similar in ability. The difference in form between these adhesives is a testament to silicone’s material flexibility; the sameness in ability, to silicone’s aptitude in extreme environments. In addition to bonding, other uses attest to silicone’s alterable and evolving canvas when it comes to ability in aerospace and aircraft applications.
Fuel- and Solvent-Resistant Silicone Elastomers
Materials that resist swell when in contact with fuels, hydrocarbon solvents, and glycols are often required to maintain the effectiveness of integral parts in air or spacecraft, and, by extension, to preserve the functionality of the vehicle as a whole. Compared to conventional dimethyl silicones, fluorosilicones provide higher swell resistance and therefore even longer reliability in prolonged exposure. Fluorosilicones are commonly used in aircraft and aerospace applications for their lack of swell in glycol de-icing fluids and fuels common to the aircraft industry, such as JP-8 and Jet A.
Figure 2 offers a visual comparison of submersion in JP-8 jet fuel for a dimethyl silicone and a fluorosilicone. Swell resistance is an ability conferred by trifluoropropyl groups on the polysiloxane polymer chain. The resulting silicones, known as fluorosilicones, are just as subject to form flexibility as silicones containing no fluoro groups. Similar to other silicones, fluorosilicones can be provided as high-consistency rubbers (HCRs), liquid silicone rubbers (LSRs), dispersions, gels and even foams. They can also be used as adhesives, molded parts or protective coatings.
Using a test method based on ASTM D 471 for swell, as well as one based on ASTM D 792 for specific gravity, researchers evaluated the percent swell of fluorosilicones vs. dimethyl silicones in JP-8 jet fuel (see Figure 3). The percent swell is depicted as the percent difference in specific gravity between t=0. Cured samples were prepared at 1 in. x 1 in. x 0.070 ft, and samples were tested for specific gravity before and after 24, 48, and 72 hours of exposure.
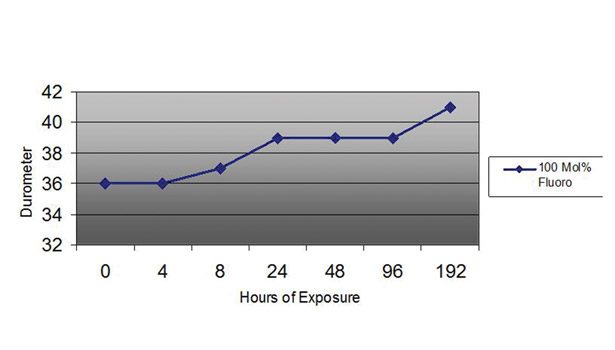
The thermal stability data displayed in Figure 4 for a 100 mol % fluorosilicone elastomer represents fluorosilicones’ endurance in harsh environments. Throughout suspension in an oven at 150°C (302°F), three specimens of this product were tested and their values averaged for durometer, Type A (ASTM D 2240) at 4, 8, 24, 48, 96, and 192 hours to determine the extent of change at these times. To be considered a passing data point, each value had to be within a 15% range of the collective median. After 192 hours’ exposure to 150°C, this fluorosilicone demonstrated adequate resistance to high temperature, with a 14% increase in durometer after eight days.
Results suggest swell resistance does not come at the price of temperature resistance. For certain silicone formulations, this environmental reliability extends to the ability to reduce the adhesion of ice to aerodynamic surfaces.
Ice-Release Silicone Coatings
Ice adhesion is a major area of significance in the aircraft industry because ice buildup affects many aspects of flying. For instance, when ice accumulates on the wings of airplanes, it decreases lift and increases drag. Wind tunnel tests have shown very thin ice sheets can reduce lift by as much as 30% and increase drag by 40%.4 Using materials or applying coatings that reduce ice adhesion to surfaces is a practical and economical choice for aircraft manufacturers, but this is a difficult undertaking, considering the adhesion strength of the ice must be less than the shear stress the ice exerts on the substrate.
In recent years, silicone coatings have gained popularity in the aircraft industry for their effective ice-release characteristics, in addition to a broad operating temperature range and resistance to many different aviation fluids. In events of ice buildup, silicone’s ability to remain elastic at low temperatures has proven extremely valuable. Silicone ice-release coatings outperform other materials commercially available and marketed as ice-release materials.
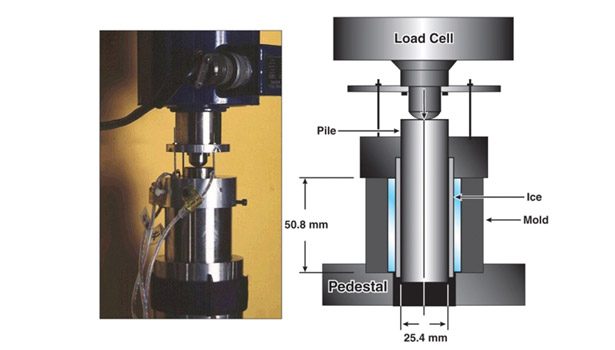
The U.S. Army’s Cold Regions Research and Engineering Laboratory (CRREL) has evaluated various materials for ice-release performance using the zero-degree cone test, which quantifies ice adhesion strength as the force required to push a coated pin from an ice mold. The test is discussed in detail in the aerospace information report (AIR) 6232. The AIR is a new publication of SAE that provides information on methods for evaluating ice-release coatings.
To begin the zero-degree cone test, water is added to a mold surrounding the coated test pin and then frozen. An O-ring placed at the bottom of the inner cylinder keeps any water from leaking out before it freezes (see Figure 5). After spending 48 hours at -10ºC, the sample is tested on the equipment with a constant rate of 0.06 mm/min. The force required to push each pin out of the ice mold is at this point quantified to determine the adhesion strength of the ice.
Figure 6 displays zero-degree cone test ice-release performance results for R-2180, R-3930, R-3975, R-1009, and R-1082, in which coatings were evaluated alone as well as in combination with R-1182, a one-part, fast-cure, room temperature vulcanizing (RTV) complementary silicone coating that prevents the silicone from being a particle gatherer. R-3930 and R-3975 are fluorosilicones, and the rest are dimethyl silicones. R-3975 had the lowest ice adhesion strength of the two fuel-resistant coatings, as well as the lowest overall result when the coatings were tested in combination with R-1182. R-3975 also showed the least discrepancy in ice adhesion strength from being tested neat to being evaluated with R-1182 coated on top.
A Versatile Solution
Silicone’s versatility makes it conducive to a wide range of applications. This capacity for optimization is why many engineers and manufacturers turn to silicone technology to serve their evolving and forward-looking innovations. Whether silicone is used as an ice-release coating to reduce ice adhesion to aerodynamic surfaces; a film adhesive for solar assembly or other applications benefiting from controlled bond line thickness; or as a fluorosilicone for swell resistance in contact with or immersed in engine fluids and other solvents, testing results show silicone is a particularly valuable asset to the aerospace and aircraft industries.
For additional information, visit www.nusil.com.
Resources
1. ASTM E 595, “Standard Test Method for Total Mass Loss and Collected Condensable Materials from Outgassing in a Vacuum Environment.”
2. NASA, SP-R-0022A
3. ESA, PSS-014-702
4. Mulherin, N.D., Haehnel, R.B., Jones J.F., “Toward Developing a Standard Shear Test for Ice Adhesion,” Proceedings, 8th International Workshop on Atmospheric Icing Structures, Reykjavik, Iceland, June 8-11,1998, IWAIS 1998.