Tack Measurement Of Heat-Activated Polyurethane Adhesives
Polyurethanes are a class of adhesive raw materials that, over the last 40 years, have developed a reputation for reliability and high performance in many applications. Properties of polyurethane adhesives can be tailor-made to fit applications due to the great variety in raw materials that can be used to formulate polyurethanes.1 As environmental legislation limits the further use of solvent-based systems, there has been rapid growth in the area of water-based polyurethanes.
Typical performance characteristics of aqueous polyurethane dispersions include the following.
- High initial and final bond strength
- Resistance to moisture and plasticizers
- Adhesion to difficult-to-bond substrates
- Ability to blend with other aqueous systems
- Ease of application by way of wet bonding or heat-activation process
Polyurethane dispersions have found applications in the automotive industry for bonding interior trim, in the furniture/construction market for laminating vinyl to medium-density fiberboard, in the shoe industry for sole bonding and in packaging applications (film-to-film, film-to-foil).
Chemistry of Polyurethane Dispersions
For most polyurethane dispersion applications, aliphatic polyisocyanates, such as hexamethylene diisocyanate, isophorone diisocyanate and dicyclohexylmethane diisocyanate, are preferred. Polyester or polyether polyols can be used to prepare dispersions, although better adhesion properties are often obtained with polyester-based dispersions. Crystalline polyester polyols are often the preferred raw materials for adhesive applications; they produce adhesive dispersions with high initial peel strength and excellent plasticizer resistance. These dispersions are favored for heat-activation bonding applications and show excellent adhesion to substrates, such as plasticized PVC and leather. The typical ionic groups used to add hydrophilicity to polyurethane polymers are based on carboxylate ions and sulfonate groups. Hydrophilicity can also be incorporated into the polymer backbone by way of polyols containing polyethylene oxide blocks.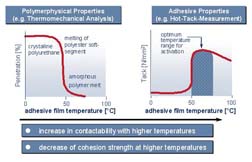
Application Conditions
Polyurethane dispersions are most often used in an application process known as the heat-activation bonding process, whereby adhesive is applied to a substrate and water is evaporated in a drying tunnel. At this point, the adhesive surface is warm and tacky, and the substrates to be bonded are mated. Pressure is applied to the bond for a short period of time by way of a fixture or a vacuum-operation to produce the finished bonded assembly.Polymers undergo a number of transitions during the heat-activation bonding process as the temperature changes (see Figure 1). The graph on the left shows a typical curve generated from thermomechanical analysis for a crystalline polyurethane dispersion. It indicates that, at a temperature of ~50°C, the polymer softens significantly. This is due to a melting transition of a crystalline polyester segment in the polymer backbone.
The graph on the right of Figure 1 refers to adhesive tack. If the tack of the adhesive film is measured at various temperatures, it is observed that, at room temperature or below, many polyurethanes form a non-tacky film similar to a coating. However, at the same temperature of ~50°C, the tack property jumps in value. This suggests that the adhesive film needs to be warmed to ~50°C before tack develops, thus enabling a good bond to form. If the film is overheated, the tack value decreases due to a further softening of the polymer.
Once the adhesive film is sufficiently heat-activated, the bonding process can proceed. The substrates are mated under pressure and the bond line begins to cool. At this point, the bond strength begins to develop. After a period of minutes or hours, depending on the adhesive used, the polymer backbone recrystallizes and a significant increase in bond strength occurs. To have a successful bonding operation, the adhesive layer must be sufficiently activated (heated), and the bonding fixture has to apply the required pressure for the necessary time period. Thus, time, temperature and pressure need to be controlled.
Polyurethane Dispersions
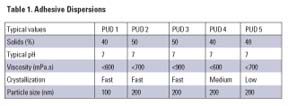
The Dispercoll U polyurethane dispersions used in this study (see Table 1) are co-solvent free and are stabilized by sulfonate groups. They are based on aliphatic polyisocyanates and polyester polyols. The products have good storage life (a minimum of six months), very good mechanical stability, are film-forming at ambient conditions and exhibit good compatibility with other polymer dispersions (e.g., VAE, acrylic).Measurement of Tack
In a previous paper, we described how the bonding process parameters of temperature, pressure and time could be modeled by an in-house developed laboratory tack-testing instrument.2 The data provided information to characterize the adhesive performance of our polyurethane dispersions and to quantify differences in their activation behavior. In this paper, tack data were generated using the TA.XT Plus Texture Analyzer (Texture Technologies Corp., Scarsdale, NY/Stable Microsystems, Godalming, Surrey, UK). This instrument has been used to measure the tack of pressure-sensitive adhesives.3-4
Experimental
Films of waterborne polymers were drawn down on glass plates at a wet film thickness of 20 mils, and were allowed to dry for two hours at 23°C/50% relative humidity before testing. The glass plates were placed on the heated testing platform and allowed to equilibrate for 5 minutes at each temperature before data was collected.A P/5 stainless steel probe with a 5 mm diameter and flat tip was used to measure tack for the films of the waterborne polymers. The probe was wiped with acetone between tests to remove any adhesive that may have transferred to the probe during testing.
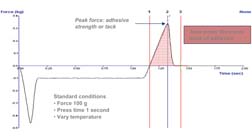
The standard test conditions used were a force of 100 g applied for a period of one second. The probe was lifted from the surface at a rate of 0.2 mm/sec. Data were generated in triplicate. The data were analyzed to determine tack (peak height) and work of adhesion (area).
The experiments to determine the rate of crystallization were carried out as follows. A dried film (20 mil wet film thickness) on glass was heated for 2 x 10 seconds in an infrared flash-heat activator (Funck Type A 2000) to achieve a surface temperature of ~55°C. The heated films were then moved to the tack tester. The tack value was measured over a period of four minutes using a force of 100 g applied for one second.
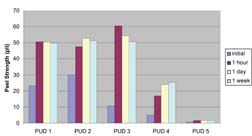
The surface of PVC containing 30% DOP (dioctyl phthalate) was roughened with a rotary sander to provide a clean surface for bonding. The dispersion was applied to PVC (7" x 8" area of an 8" x 8" panel) and allowed to dry for one hour at room temperature. The panel was cut in half and the surfaces were activated to a surface temperature of 65°C with an infrared flash activator. The pieces were pressed together for fifteen seconds at a pressure 200 psi and cut into 1" wide strips for testing. The peel strength was measured initially, after one hour and after one day, at a pull rate of 4"/min.
Results and Discussion
Tack Behavior of Polyurethane Dispersions
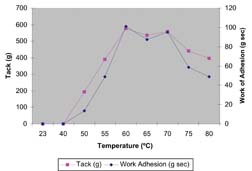
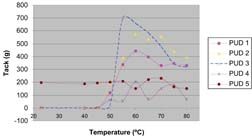
Figure 4 shows the tack vs. temperature profile for a polyurethane dispersion designed to be used in a heat-activated adhesive application. The graph shows the tack and work of adhesion. The material has essentially no tack at low temperatures (45°C) and exhibits the behavior of a dry non-tacky film. At a temperature of ~50°C, the tack becomes measurable and has a peak value at ~60°C. At temperatures >70°C, the tack value decreases since the viscosity of the polymer decreases at higher temperatures, which leads to a lower value of cohesive strength of the bond. The graph also shows that work of adhesion also reaches a peak value at ~60°C. It is known from DSC measurements that PUD 2 has a melting transition at a temperature of 48°C. Thus, for this polymer, which has a crystalline backbone, tack increases significantly after the polymer backbone undergoes a melting transition.
Recrystallization Rate of a Polyurethane Dispersion
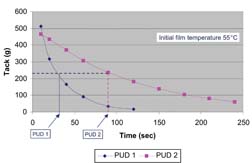
A critical property of polyurethane adhesive dispersions is its recrystallization rate. It is has been found that dispersions that crystallize rapidly will show a high initial green strength value. The tack test instrument was used to determine the crystallization rate of dispersions PUD 1 and PUD 2. The films were heat-activated to a temperature of 55°C and the tack was measured initially and as the film cooled. The results are shown in Figure 7. The graph shows that PUD 1 loses 50% of its initial tack value in ~27 seconds while PUD 2 takes ~80 seconds to decrease to this level of tack. Thus, an operator would have a longer time to adjust placement of a substrate if PUD 2 were used, and could expect a faster development of bond strength if the adhesive was based on PUD 1.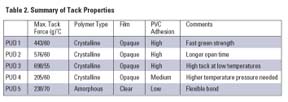
Summary
Thermoactivatable adhesives, based on polyester polyurethane raw materials, have a long and successful history in adhering difficult-to-bond substrates. The products described in this paper are particularly advantageous since they contain no co-solvents.High-performance adhesive systems can be based solely on polyurethane dispersions, or the cost/performance ratio of an adhesive can be optimized by blending polyurethane dispersions with other polymers. The dispersions presented allow the formulation of adhesives with low heat-activation temperature that provide high initial and final bond strengths. Due to their neutral pH, they have a wide compatibility with other polymer types. Optimal heat resistance is obtained by crosslinking these adhesives with a water-dispersible polyisocyanate. A typical starting formulation for a polyurethane-based adhesive is shown below.
Polyurethane dispersion.......100 parts
Thickener........................0-2 parts
Blending polymer..............optional
Polyisocyanate crosslinker...3-5 parts
The tack character of these dispersions is an important variable that impacts adhesive performance. Through an understanding of the degree of tack of a system and its temperature dependence, it is possible to make more informed recommendations on which raw material is best for a specific set of application conditions. The techniques reported in this paper offer a method to quantify the tack performance of polyurethane dispersions, as well as blends with other polymers.
For more information, contact Jeffrey F. Dormish, Bayer MaterialScience LLC, 100 Bayer Road, Pittsburgh, PA 15205.
References
1. Schmelzer, H.G. 1987. Polyurethanes World Congress, 614-25.2. H.-W.Lucas, H.W. 1996. Journal of the Adhesives and Sealants Council Volume 1, 237 - 257.
3. Chuang, H.K.; Chiu, C.; Paniagua, R. 1997. "Avery Adhesive Test Yields More Performance Data Than Traditional Probe," Adhesives Age, September, 18-23.
4. Johnson, B. 2000. "Tape Measure: New Methods for Evaluating the Physical Properties of PSA Tapes," Adhesives Age, July 2000, 40-45.
5. TA.XT2 Texture Application Study: Pressure Sensitive Adhesive Tapes Study Number I-3W. Texture Technologies Corp.
SIDE BAR: Abbreviations used in text and graphs
PUD 1: Dispercoll® U 53PUD 2: Dispercoll® U 54
PUD 3: Dispercoll® U 56
PUD 4: Dispercoll® U KA 8713
PUD 5: Dispercoll® U KA 8758
Registered Trademarks of BAYER AG,
Germany: Dispercoll®
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





