Surgery Sealant
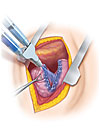

The DuraSeal technology is a patented synthetic, absorbable hydrogel delivered by a dual syringe applicator. The device can be stored at room temperature and prepared in less than two minutes. When sprayed on the pleura, a strong, adherent hydrogel is formed that effectively seals the suture or staple line within seconds. The sealant features a blue colorant that provides the thoracic surgeon with visualization of coverage and thickness of the material upon application to the lung. Post-operatively the sealant continues to seal the resected site as healing progresses under the gel. After several weeks, the hydrogel breaks down into water-soluble molecules that are absorbed and cleared through the kidneys.
Larry Kaiser, M.D., chairman of the Department of Surgery at the University of Pennsylvania, said, "I have worked with other sealants in the past. However, they have not been easy to use and this has limited their adoption. DuraSeal is unique in that it is not only synthetic and elastic, which are important attributes for a lung sealant, but it is also extremely easy to prepare and use. This could become an important tool in helping the thoracic surgeon reduce or eliminate air leaks."
"Each additional day that the patient spends in the hospital due to prolonged air leak can mean thousands of dollars in added expense. An effective lung sealant that can reduce the incidence of prolonged air leak will be a tool that surgeons and hospitals will enthusiastically embrace," said Bryan Meyers, M.D., Division of Cardiothoracic Surgery at Washington University in St. Louis.
"With this approval, DuraSeal will be the only synthetic surgical sealant approved for use in thoracic surgery in the European market," said Amar Sawhney, Ph.D., co-founder, president and CEO of Confluent Surgical. "We have been pleased with the successful reception of DuraSeal in neurosurgery and look forward to introducing this unique product to the thoracic surgical community."
Approximately 300,000 lung resection procedures are performed worldwide each year, and about 80% of these result in at least some level of air leak.
Confluent Surgical currently markets the DuraSeal Dural Sealant System for use as an adjunct to sutured repair of the dura in several countries, including the U.S., and has established an extensive and distinguished neurosurgeon-focused distribution channel to represent the DuraSeal product in the U.S. market.
Confluent Surgical is a private medical device company that is pioneering the development of in-situ-polymerized biomaterials. The synthetic materials are safe, simple to use, and allow the formation of customized implants at the site of disease. These materials show potential for numerous applications across several surgical disciplines.
For more information, contact Patrick O'Donnell, director of Global Marketing, at podonnell@confluentsurgical.com , or visit http://www.confluentsurgical.com and http://www.duralsealant.com .
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



