Dipodal Silanes


Dipodal silanes have been successfully used as adhesion promoters in multi-layer printed circuit boards. Photo courtesy of Philips.
Adhesives and sealants play a vital role in commercializing these technologies. However, in order for adhesives to be accepted as a material for these applications, two factors must be considered: assembly of complex devices and the long operating lifetime in the aggressive environments where the devices must perform. From an economic “cost of ownership” model, adhesives reduce intense fabrication costs or enable the assembly of these complex devices. Extending the operating lifetime of these devices is equally important. Requirements for the initial assembly (temperature limitations, environmental regulations and safety requirements) handicap the adhesive formulator in achieving durable chemical bonds in aggressive environments. A key element in long-term adhesive failure is insufficient hydrolytic stability at the bond line.
Dipodal silanes are a new series of adhesion promoters that have achieved commercial success in applications that include plastic optics, multi-layer printed circuit boards and use as adhesive primers for ferrous and nonferrous metals. These products have intrinsic hydrolytic stabilities up to ~10,000 times greater than conventional silanes. They have a significant impact on substrate bonding and the mechanical strength of many composite systems, including epoxy, urethane, epoxy/urethane hybrids, polysulfide, cyanoacrylate, and silicone, and may also be used in waterborne, high-solids, and photoactive chemistries.
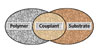
Figure 1.
Conventional Silane-Coupling Chemistry
RnSiX(4-n) is the empirical formula for organosilanes and illustrates the silane’s reactive abilities. The R group is a nonhydrolyzable organic radical capable of bonding with organic resins and polymers. The X group is hydrolysable (typically alkoxy, acyloxy or chlorine) and enables the silicon group to bond with inorganic substrates. Thus, an organofunctional silane that is capable of reacting with both organic (R group) and inorganic (X group) substrates can function as a bridge between the two (see Figure 1), such as between an inorganic mineral and a polymer and/or between a polymer and a solid surface.
Figure 2. Reactions Involved in Silane Coupling
The reaction of silane-coupling agents involves five basic steps.
1. Hydrolysis generates reactive silanol groups, which are the bonding sites for the attachment to inorganic substrates.
2. Condensation to oligomers follows hydrolysis.
3-4. Oligomers hydrogen bond with available hydroxyl groups of the inorganic surface (functional fillers, metal oxides) to form stable siloxane linkages.
5. The reactive organofunctional group (usually amine, vinyl, mercapto, methacrylate or epoxy) will then form covalent and hydrogen bonds with the organic resin or polymer during cure.
The reactions involved in silane coupling are shown in Figure 2.

Figure 3. Dipodal Silane
Dipodal Silanes
Due to the nature of the silicon molecules, the silane-coupling agent is a material used to resist deterioration by the intrusion of water between the polymer and the substrate. Through the modification of the interface, silane-coupling agents not only provide water resistance, but are also responsible for other important changes associated with composite systems. The interface region may exhibit increased strength because of the modification, which forms interpenetrating polymer networks of resin and silane.In silane surface treatment or in situ applications, it is common to hydrolyze the alkoxy groups to form silanol-containing species, which are highly reactive and responsible for hydrogen bonding with the substrate. However, it would be ideal to supply silanes with enhanced hydrolytic stability.
The problem with conventional silanes is that they self-condense to form siloxanes, resulting in phase separation or gelation. By adding dipodal silanes, the enhanced hydrolytic stability will have significant impact on shelf life, substrate bonding and improved mechanical strength of many composite systems.
Functional dipodal silanes and combinations of non-functional dipodal silanes with functional conventional silanes have a significant impact on substrate bonding and possess enabling activity in many adhesive systems, particularly primer and aqueous immersion applications. The fundamental step by which silanes provide adhesion is forming a –Si-O-X bond with the substrate. If the substrate is siliceous, the bond durability is dictated by bond dissociation of Si-O-Si. According to the equation ≡Si-O-Si≡ + H2O ⇌ ≡Si-OH + ≡Si-OH, the equilibrium for bond dissociation is ~10-2. By increasing the number of bonds by three, the equilibrium for dissociation is increased to ~10-6.
Theoretically, this means that the dissociative bond line failure that typically occurs in one month is increased to ~10,000 months. Other factors influence the failure, but dipodal silanes clearly have the potential to exceed the lifetime bond requirements of many devices. The effect is thought to be a result of both the increased crosslink density of the interphase and the resistance to hydrolysis of dipodal silanes, which is estimated at ~10,000 times greater than conventional coupling agents. Dipodal silanes have the ability to form six bonds to a substrate, compared to the three bonds of conventional silanes.

Table 1. Non-Functional and Functional Dipodal Silanes
- Improved wet adhesion
- Improved chemical resistance
- Corrosion protection
- Improved processing.

Table 2. Effects of Dipodal Silane on the Bond Strength of Crosslinkable Ethylene-Vinyl Acetate Primer Formulation
Conclusion
Conventional silane and dipodal silane chemistry has been reviewed. The theoretical information presented with the empirical data substantiates that a combination of dipodal and conventional silanes offers enhanced bond strength vs. conventional silane-coupling agents. Dipodal silanes, when used in adhesive and sealant formulations, offer performance advantages as well as enhanced shelf-life stability, which will allow formulators to meet the requirements set forth by future high-performance applications.For more information, contact Gelest Inc., 11 E. Steel Road, Morrisville, PA 19067; phone (215) 547-1015; or visit www.gelest.com.
Links
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!


