Q&A About Polyurethanes
Can you explain the chemistry behind polyurethane dispersions?
Solventborne polyurethane adhesives have been used for over 50 years. They have a long history of producing high-strength, durable bonds, and can be used on a range of substrates. As industrial hygiene concerns in the workplace have grown, an effort has been made to eliminate solvents when possible. Polyurethane dispersions have been developed that have excellent adhesion performance and effectively address the safety aspects raised by the use of solvents.
The backbone of a polyurethane dispersion will have the same basic composition as the solvent-based polymers, but will contain hydrophilic groups that allow the polymer to be dispersed in water. The dispersed particles consist of a hydrophobic core surrounded by a hydrophilic shell composed of ionic groups and/or long-chain hydrophilic nonionic groups. In contrast to other types of dispersions, polyurethane dispersions most often contain only internal emulsifying groups, meaning they are built into the polymer backbone. Other dispersions, such as polychloroprenes, are stabilized by the addition of external emulsifiers. Ionic or nonionic surfactants that are not chemically attached to the polyurethane polymer could migrate out of the adhesive and lead to bond failures.
Since the hydrophilic groups remain in the polymer film, water swell resistance and hydrolytic stability decrease with increasing amounts of internal emulsifiers. The goal is to add a sufficient number
of hydrophilic groups to produce a stable dispersion and avoid using an excessive amount, which could negatively influence the hydrolytic stability of the end product. A well-formulated dispersion will contain enough hydrophilic groups to produce
a dispersion with a particle size of
100-300 nanometers.
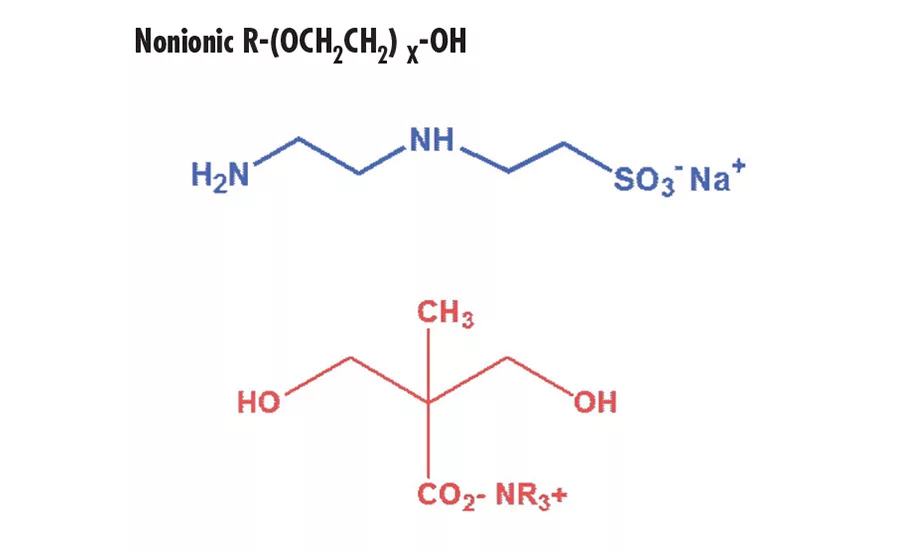
Typical hydrophilic groups are shown below:
Nonionic R-(OCH2CH2) X-OH
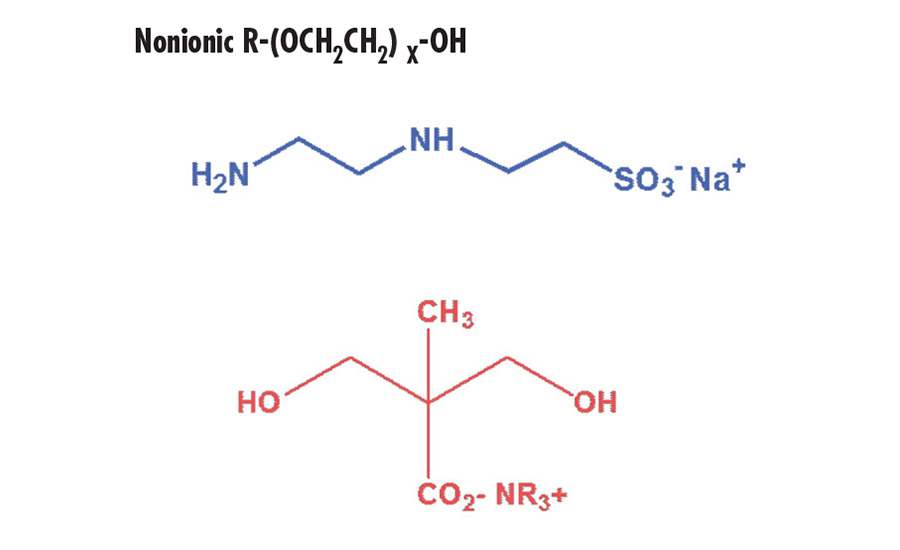
Dispersions containing sulfonate groups have a pH value of approximately 7 and are stable at both higher and lower pH values. This allows for significant formulating flexibility when blending with other polymers. In contrast, carboxylate stabilized dispersions have a higher pH
(> 8). They are unstable at lower pH values, which limit formulating options. Nonionic compounds containing multiple segments of polyethylene oxide can also be used as hydrophilic modifiers.
Polyurethane dispersions with ionic stabilization may coagulate when exposed to metal salts or freezing temperatures. Freeze/thaw-stable dispersions exist, but they often contain significant quantities of cosolvents. Dispersions containing solely nonionic hydrophilic groups have stability issues at elevated temperature (> 70°C). Once dispersions coagulate, they cannot be redispersed.
To develop the best properties of the adhesive, it is often recommended to use the dispersion in conjunction with a crosslinker. Water-dispersible polyisocyanates are a popular class of crosslinkers. They will react with hydroxyl groups on the polymer backbone and increase the polymer’s molecular weight and crosslink density. Polycarbodiimide crosslinkers are used to cure polyurethane dispersions containing carboxyl functionality.
Having a good understanding of the chemistry behind an adhesive is extremely useful for formulating a product with high performance.
Author's Note: I have retired from Covestro LLC, and this will be my last column in ASI magazine. Thank you for your interest in polyurethane chemistry over the past eight years. I hope you will continue to develop high-performance adhesives based on polyurethane chemistry. My current email address is jdor_adh@outlook.com.
Any views or opinions expressed in this column are those of the author and do not represent those of ASI, its staff, Editorial Advisory Board or BNP Media.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



