Titanium Dioxide Pigment Post-Treatment: Influence on Electrostatic Stability of Technical Dispersions
The surface chemistry of titanium dioxide dispersions can be controlled via specialized measurements to reduce agglomeration.

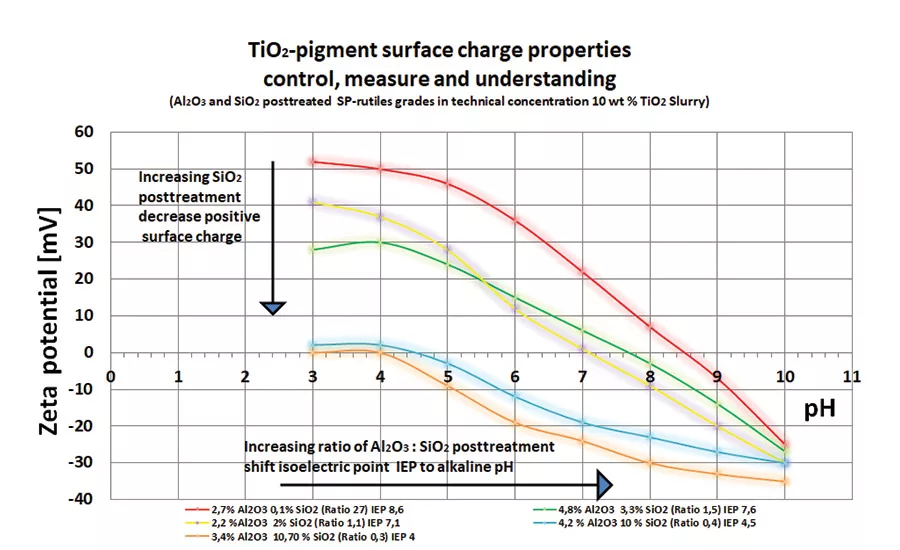
Figure 1. ESA results.

ESA measurements of highly concentrated dispersions are special testing methods that have been developed for a range of different applications.
Titanium dioxide (TiO2) can be used as a pigment base in applications such as coatings, plastics, and laminates. No matter the application, it is important to avoid the coagulation and agglomeration of the TiO2 dispersion during both the pigment production process and the end-use manufacturing process.
The surface chemistry of TiO2 dispersions can be controlled through the measurement of electrokinetic-sonic-amplitude (ESA) by regulating the pH value (e.g., for avoiding flocculation of the dispersion). This means that the zeta potential of the dispersion should not be or close to zero. Zeta potential is a dimension of the effective surface charge of particles and the interaction between the particles with ions in solution and particles among each other.
Characterizing the absolute surface charge (in an aqueous dispersion) of the TiO2 pigment, negative or positive in the unit (mV), gives a decisive parameter for the ultimate application of the product. The zeta potential depends on the kind of solvent, the nature and amount of the ions in solution (specific conductivity), and the pH value. It is also the main factor that determines the stability of the whole dispersion.
Testing Materials and Procedure
Measurement of TiO2 dispersions was carried out using five TiO2 pigment grades with different post-treatments in aqueous dispersion. Materials included an aqueous TiO2 dispersion 10% wt, 0.1 M HCl, and 0.1 M NaOH (p.A.). Testing was performed with an ESA measuring system incorprating a programmable titrator. A hypersensitive probe with 500 kHz was used to measure frequency. The system also included an ESA measuring cell, pH sensor, conductivity and temperature sensor, and an adjustable stirrer to prevent precipitation during measurement.
Results and Discussion
Measurements using the ESA deliver a clear distinction of the electrostatic properties of various grades of TiO2 in aqueous dispersions. The measurement results in Figure 1 show that various TiO2 grades differ significantly from each other in isoelectric point (IEP) and with regard to their zeta potential. Handling TiO2 dispersions in a range beyond the IEP avoids any kind of flocculation.
Choosing pH values in an extreme area could be a disadvantage regarding both the electrostatic property of the pigment surface and the ionic charge in the dispersion. With an increasing ratio of Al2O3/SiO2 post-treatment of TiO2 base material, the isoelectric point IEP shifts to alkaline pH values. With increasing SiO2 post-treatment, the positive surface charge decreases.
Experienced users and specialists know well that Al2O3 and SiO2 compounds have been used for decades in TiO2 post-treatment processes. However, improvements have been made in the type of post-treatment, such as in the targeted control of shell texture (dense, continuous), structure (heterogeneous, homogeneous), layer thickness, etc.
Specialized Measurements
ESA measurements of highly concentrated dispersions are special testing methods that have been developed for a range of different applications. The added value of the current study lies in using measurement systems for highly concentrated technical formulations. During technical processes, dispersions are often found to be highly concentrated, muddy, colored, tempered, or electrostatically affected by additive formulations. Sedimentation is often inhibited by powerful agitators in large-scale treatment containers. All of these process conditions have been reported in the stability analysis shown here.
The surface chemistry of TiO2 dispersions can be controlled during the measurement by regulating the pH value, adding additives, and stirring using the same conditions as those used during manufacturing processes. The results of the analyzed, highly concentrated samples correlate directly with the electro-kinetic properties of the dispersed particle in both the raw material and the final product.
For additional information, visit www.tinoxchem.de.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






