Advancing Adhesives
Advancing Silicone-Based Medical Adhesives
A new adhesive for wearable medical devices enhances patient comfort while providing engineers confidence about wear duration.
Acrylate and silicone have dominated the medical adhesives market for years. However, the adhesives currently available often require device engineers to choose between strength and wear duration, or comfort and pliability. To address these issues, 3M developed what the company is calling the next generation of its silicone adhesives, 3M™ Hi-Tack silicone adhesive tapes.
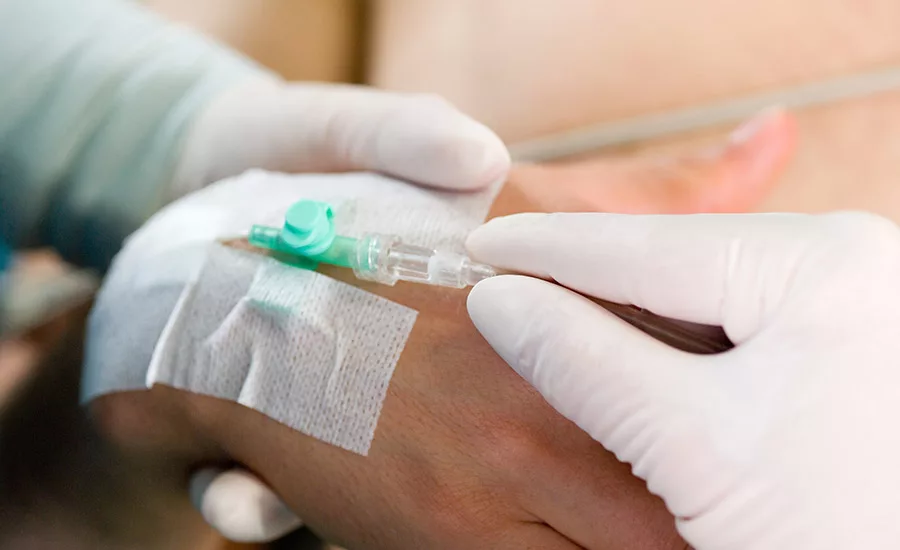
3M reports that the new 2480 3M Single Coated Medical Nonwoven Tape with Hi-Tack Silicone Adhesive on Liner provides increased sheer performance, higher tack, stronger adhesion, and longer wear duration. The adhesive is repositionable, flexible, and conformable to work well with various medical devices, including continuous glucose monitoring systems, wearable monitors, and sleep and incontinence devices. It is strongly bonded to the backing to minimize residue on both skin and production equipment, and it is compatible with ethylene oxide (EtO) sterilization.
ASI Editor Susan Sutton recently spoke with John Dugas, global portfolio manager for 3M Medical Materials and Technologies, about the new adhesive, as well as the various factors involved in product development for adhesives used in medical applications.
What are some of the special considerations that adhesives producers need to take into account when focusing on medical end uses?
It’s really complex. You have to consider regulations. You need to consider if you can even manufacture the device and use it appropriately when you’re making it. But by far, the biggest consideration is patient safety when we’re dealing with adhesives in the medical space. And there’s a couple of factors within patient safety that make it critical.
First of all is the device itself. You have to work with the device so it’s safe. But second, and I will say more important, is making sure you’ve got the right design and the right adhesive on there to minimize any unintended effects. If you go in for a cardiac monitor, you don’t want to come out of the hospital with irritated skin. Poor device and adhesive compatibility can turn people away from treatments and keep them from getting the appropriate regimens.
That’s why we’ve been working a lot on this different class of adhesives that are gentler, which helps reduce the aspect of skin trauma and discomfort from the patient equation so that they can really focus on the treatment themselves and the devices themselves, not the ancillary parts like the adhesive holding it in place.
What were your main goals when you were in development for this new product?
I’ll go back, first of all, to patient safety—to come out with the gentlest adhesive to minimize skin trauma and discomfort as we could. The other aspect is we’re seeing a lot of trends in the medical marketplace, especially in the wearable or monitoring market. I’ll give you a really good example. Healthcare data is one of the fastest growing areas in healthcare. And what that data does is it helps enable better patient treatment paths.
Think about diabetes, which is one of the fastest growing chronic diseases on the planet. We used to manage diabetes by pricking your finger two or three times a day to get your blood glucose level. We had three points of data, and you’d adjust your blood glucose three times a day. So you could spike really high. You could go really low. Now, the new glucose monitors are held in place on the skin, and they’re continuously monitoring them so they can make little adjustments instead of big swings. Think about body temperature for COVID patients. You want to continuously monitor temperature, so you’ve got sensors that you wear 24 hours a day to monitor how it goes up and how it goes down. Blood oxygen is another thing. The role of healthcare data and monitoring is just growing really fast.
As we’re looking at adhesive challenges out there, we have to come up with adhesives that plan for that trend and additionally support capturing that information. Devices are getting more complex, they’re getting heavier. They’re going on different parts of the body. Whether you’re monitoring brainwaves, glucose, heart conditions—they’re going all over the body. We really focused on that gentle adhesion, but at the same time finding the right adhesive that increases wear-time and can hold on those heavier and more complex devices for the time they want.
We don’t always have the ability to change the device every hour. Medical professionals might want to put something on that’s a complex device with the appropriate battery to monitor that patient without having to go and check their temp or move it around or replace it every hour. This is why we focused on adhesion and wear time, which is where high-tech silicones come in. They’ve got the gentleness of silicones, but they’ve got the holding power and wear duration that you expect for more complex monitoring devices.
![]()
The adhesive is repositionable, flexible, and conformable to work well with various medical devices, including continuous glucose monitoring systems, wearable monitors, and sleep and incontinence devices.
What are some of the other factors that need to be taken into consideration when developing these types of wearable medical devices?
A really good example is repositionability. When you’re talking about the increase in the monitoring market, especially in neurological applications or heart monitors, you may have to find a specific spot on your body to be able to get the right signal. So being able to reposition a device may be really critical for your product.
I think the second thing that we’re really looking at and driving for is the adhesion level, because devices are being worn for longer periods of time. To satisfy that longer wear time, you have to look at different types of adhesion; adhesion is just not one concept. You can have tack from an adhesion standpoint. How sticky is it? Can I put it on and it stays in place so I can get that original signal and move it around? We also spent a lot of time on shear. If you’re walking around all day and you’ve got a device on your body, you have to make sure it doesn’t slide down your body, which is the shear force in your adhesive.
I think the last thing I’d like to mention that we forget about as you’re talking to adhesive and device manufacturers is manufacturability. Sometimes we don’t consider the manufacturability of the product. When you’re looking at new areas or new materials, you forget that someone’s going to have to manufacture it. Consider sensors as an example. Some companies are already manufacturing millions and billions of sensors, and you can’t be cutting those out individually or running a slow process.
We did spend a lot of time with these new adhesives, focusing on making sure, for example, that they’re really thin so they conform to your manufacturing process, or don’t apply too much adhesive. Also, we made sure it bonds well to the liner because you don’t want to run for 30 minutes and then have to shut down, clean up, and then run again. We have to make it easy for companies to manufacture, because if we can’t get the product out the door, you can’t help the patient out.
You might have the best product in the world, but if they can’t actually apply the product, then it’s not doing anybody any good, right?
Absolutely. There’s so many things going on in healthcare with COVID, and it’s just changed the industry. With these monitoring devices, we’ve seen an explosion in the number of devices that people are rolling out quickly to make it work. For example, with protective equipment, adhesives can help hold some devices in place. There’s a lot of opportunity and growth out there just based on the changing market now. And we’re all trying to help out with so many unique things going on.
Listen to our full conversation with 3M’s John Dugas at www.adhesivesmag.com/podcasts. To learn more, visit www.3m.com/scienceofsilicone.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





