Advancing Adhesives
Recycling Plastic Waste into Custom-Made Polyols
The chemical recycling of polyurethane (PU) and polyethylene terephthalate (PET) from waste materials is evolving into a viable alternative to mechanical recycling.

Old mattresses, insulating material, furniture, and steering wheels—these raw materials are not particularly appealing. But the solvolysis process is enabling Rampf Eco Solutions GmbH & Co. KG to recycle these types of waste materials and create customized polyols for applications ranging from foams to adhesives.
During the process, solvents are used to split the polymers (e.g., the urethane group in the case of PU). The processes differ in the splitting medium applied. Water is used for the hydrolysis process, while acid is used for acidolysis. These processes result in various polyols with different properties. The most suitable polyols for a required application area can therefore be precisely created by selecting the corresponding process.
The polyols produced in this way are then returned to the customer’s production facility either by Rampf Eco Solutions or another PU system company. Due to the renewed crosslinking, the materials reobtain their initial properties. In this way, a recirculatory system is created that offers combined economic and ecologic advantages.
Process Details
As mentioned, Rampf relies on the glycolysis and acidolysis processes for solvolysis. With glycolysis, the urethane group is split by means of transesterification, mostly with bivalent alcohols (see Figure 1). The process can be used for practically all polyurethanes, although the hydroxyl value (OH value, which indicates the number of hydroxyl groups in a substance) is increased during the reaction. It even exceeds that of the initial polyol, which leads to a higher hardness of the end product. Therefore, polyols obtained with the glycolysis process are mainly used for the production of hard foams, hard integral foams, and durable integral foams.
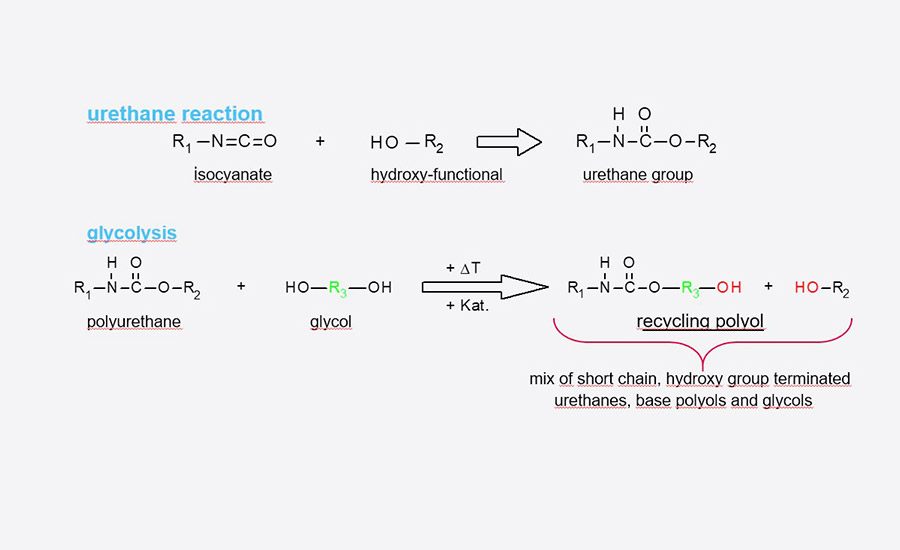
Figure 1. Glycolysis of polyurethane.
In contrast, the acidolysis process splits the urethane group with acids or acid anhydride (see Figure 2). Compared with glycolysis, the acidolysis process is more complex and more expensive. Because it does not increase the OH value, the acidolysis process is used mainly to obtain polyols for soft foam production.
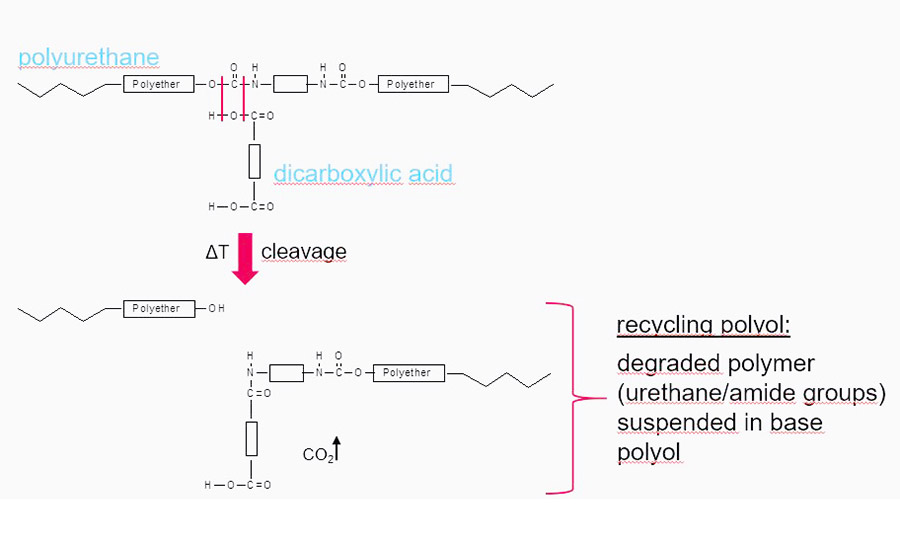
Figure 2. Acidolysis of polyurethane.
Neither process involves downcycling; they are self-supporting upcycling processes with a high added value. For example, adding recycled polyols can improve the compressive strength of insulating foams, the chemical resistance of casting compounds, or the compatibility of polyurethane systems. Consequently, the polyols can be used for the production of numerous end products.
Specially Customized Polyols
Other polymers are also suitable for chemical recycling. In 1999, for example, Rampf Eco Solutions developed a process for chemical recycling of PET together with the Deutsche Gesellschaft für Kreislaufwirtschaft und Rohstoffe (German Society for Circular Economy and Raw Materials) in Cologne, Germany. The resulting polyols are mainly suited for the production of hard foams. High mechanical strength and improved flame-proofing are the properties that are influenced decisively by these aromatic polyester polyols.
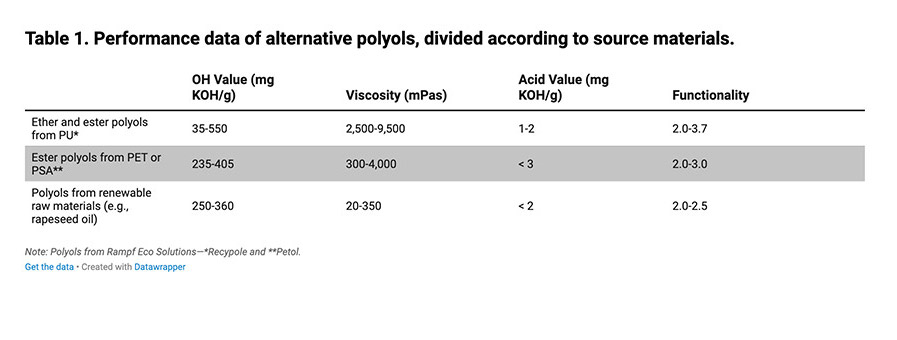
Polyesters such as polylactide (PLA, polylactic acid), polycarbonate (PC), and polyhydroxyalkanoate (PHA) are used as raw material for producing recycled polyols. Chemical recycling of PC produces aromatic polyester polyols with rigid and strong bisphenol A structural elements, making them suitable for mechanical reinforcement. The compressive strength of polyisocyanurate foams (PIR) could be improved significantly by adding PC polyols. In contrast, polyols made of bio-based PLA are purely aliphatic and very flexible. By adding these polyols, soft segments can be integrated in PU, reducing the embrittlement tendency of strong, hard foams and casting compounds (see Table 1).
Compared with mechanical recycling, the application of functioning chemical recycling processes is limited, although they are a good supplement for existing recycling systems (particularly for plastic waste that is difficult to recycle). For example, recycling bulky waste still represents a great challenge. More than 60% of the estimated 19 million tons of furniture, mattresses, upholstery, textiles, and plastic garden furniture that are thrown away in European countries every year ends up in landfills.
The Urbanrec project was organized to improve the logistics, disposal, and reuse of bulky waste (see Figure 3). Companies and organizations in seven countries participated, including national institutions in Belgium, Poland, Spain, and Turkey. The aim was the development and implementation of an ecologically effective and integral disposal system for bulky waste in order to promote waste prevention and recycling, combined with improved logistics.
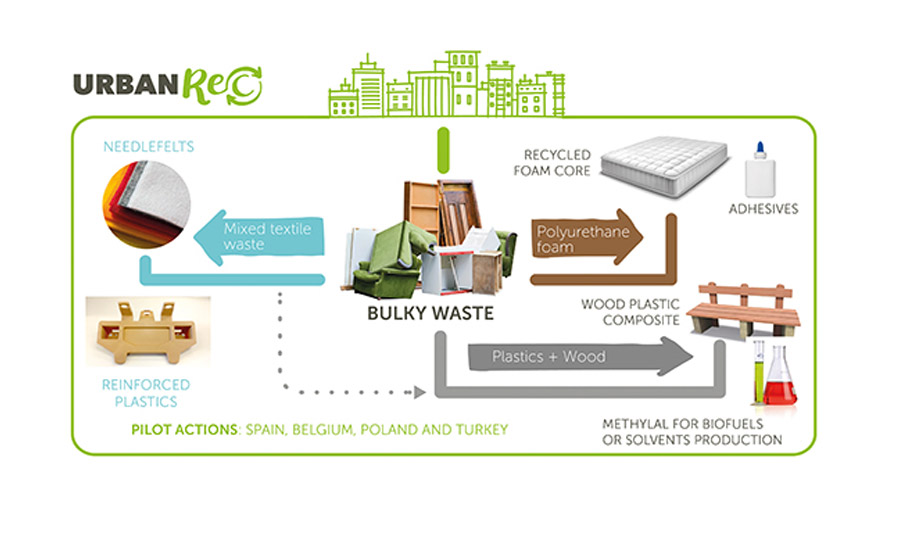
Figure 3. The Urbanrec project was organized to improve the logistics, disposal, and reuse of bulky waste.
Chemical Recycling in Practice
Within the Urbanrec project, and together with partner companies and institutions, Rampf Eco Solutions had the task of developing chemical solutions for obtaining high-grade recycled polyols from plastic waste such as mattresses and upholstery. The aim was to reduce the amount of waste delivered to landfills and incineration plants. The polyols involved were produced with the glycolysis and the acidolysis processes, and new products based on the recycled raw materials were developed during the project, including adhesives, foams, and insulating materials.
The glycolysis polyols were used to produce insulating foams with a content of up to 50% recycling polyol. Acidolysis polyols were used in the top layers of viscoelastic mattresses (12% recycling content) and for PU hot-melt adhesives (up to 50%). It became clear that separation of the PU foam qualities—ether or ester foams based on methylene diphenylisocyanate (MDI) or toluene diisocyanate (TDI)—was necessary to ensure high-quality polyols. Separation was done by means of near-infrared spectroscopy.
Solvolysis as an Alternative
Solvolysis is a suitable process for obtaining chemical raw materials from recycled PU and PET. Depending on the plastic waste and the required properties of the end product, the glycolysis or the acidolysis process is suitable.
Chemical recycling is gradually evolving into an alternative for mechanical recycling. Increasing prices for petrochemical products, tighter environmental regulations, and changing sustainability mindsets of consumers make it attractive. Moreover, projects such as Urbanrec illustrate the potential opportunities of chemical recycling in practice.
For more information, visit www.rampf-group.com.
Note: Images courtesy of Rampf Eco Solutions GmbH & Co. KG.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







