Innovative Developments for UV Silicone Release Coatings

Both methods can be used to produce release coatings with special release forces, which can be adjusted by the degree of modification of the silicone backbone. Figure 2 shows the correlation between the release force and the ratio of modified to unmodified units in the silicone backbone. Epoxy silicones have a lower release force than silicone acrylates with a similar degree of modification due to their lower polarity. Polar organic residues linked to the silicone backbone have a higher interaction with the adhesive component.

The main disadvantage of the radical curable system is the need to inert the coating unit with nitrogen because the presence of oxygen will lead to the termination of the polymerization. Therefore, the laminate must be completely cured before it leaves the inerted UV unit. This requires some technical effort. However, inerted UV units have been state-of-the-art for more than a decade. Their reliability has been proven both in production and for consistent quality of free-radical-cured silicone coatings.

As a consequence, the cure speed of cationic-curing silicones can be very different on different substrates and at different curing conditions. Thus, the degree of curing in the moment of rewinding the web may not have reached a sufficient degree. The silicone continues to post-cure on the rewind reel, which may have a negative impact on the release level and silicone transfer. Since the properties of the liner change with time, in-line processes are difficult to realize with cationic curing silicones.
There are two different curing mechanisms for the UV curing of silicone release coatings. Based on the properties of these mechanisms, different photoinitiators must be used. Cationic initiators are more expensive than the radical initiators. On the other hand, epoxy silicones are easier to manufacture than silicone acrylates and thus can be offered at a lower cost.
An advantage to the radical-curable system is the much longer shelf life with the photoinitiator included. Radical-curable systems can be stored for weeks and even months, whereas epoxy silicones can be stored only for a few days.
Specific actions that can be employed to reduce various disadvantages in each system will be discussed.
Improvement of the Cationic Curable System
Modification of the Photoinitiator1-2The main disadvantage of the cationic curable system is the slow polymerization of the epoxy functional groups compared to acrylate groups. In particular, the solubility of the photoinitiator within the system influences the speed of curing.
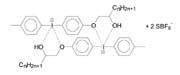
Iodonium salts are very effective photoinitiators and are suitable for the curing of epoxy silicones. The problem is finding commercially available iodonium salts that are soluble in epoxy silicones. Presently, there are various salts available on the market that vary by the nature of the substituent at the phenyl group and the type of counter ion.
These structural features influence the compatibility of the iodonium salt within the cationic-curable system. For example, iodonium salts with hydroxyl groups have an increased tendency to crystallize (see Figure 4). This leads to a limited compatibility of such materials in epoxy silicones.
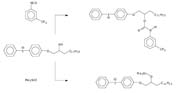
Figure 5 shows two reaction schemes that fulfill these requirements. Formula A shows the reaction of an iodonium salt with an isocyanate. The advantages of this process are that the isocyanate is relatively inexpensive and that the reaction to complete conversion to the targeted product is fast. The same is true for the reaction illustrated in Formula B. Here, a hydroxyl group is converted into a "siliconophil" residue with trimethylchlorosilane.
Both of the photoinitiators illustrated in Figure 5 are high-viscosity liquids at room temperature. They are completely compatible with white spirit, whereas the compound containing the hydroxyl functional group is crystalline with a melting point of 91
We evaluated alternative solvents with low VOC and reduced environmental hazards. We identified hydroxy functional siloxanes to be an efficient solvent and co-reactant to iodonium-based photo-catalysts. They are high-molecular-weight, low-viscosity materials that efficiently dilute the photo-catalyst and blend perfectly with the epoxy functional siloxanes. Such a photoinitiator is available now.
Modification of the Epoxy Silicone3
Modifying the silicone is another way to improve the compatibility between the epoxy silicone and photoinitiator. Patent EP 334 068 describes how the solubility of iodonium salts is enhanced when a certain fraction of the epoxy functional groups has been esterified with benzoic acid.4 However, this procedure leads to the possibility of polymerization and the loss of the comparatively expensive epoxy functional groups.
Modification with alcohol groups is a cheaper and safer method to improve the polarity of the silicone. The alcohol functional groups participate in the polymerization and cause a chain transfer reaction. This also has the positive effect of making the iodonium salts more soluble in the silicone polymer. However, it is important that the alcohol-functional groups are evenly distributed within the siloxane backbone. The same effect cannot be observed if a mixture of OH-functional siloxane and epoxy siloxane is used.
Improvement of the Free Radical-Curable System
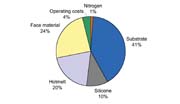
Improvement of the Oxygen Tolerance of Silicone AcrylatesThe main reason customers hesitate to use the radical-curing system is the necessity for inertization of the coating unit with nitrogen even though the cost of the nitrogen represents only 1% of the total cost to produce the laminate (see Figure 7).
We have developed a method that allows relatively high oxygen concentrations during the production process of the laminate.
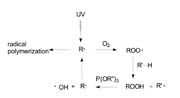
The class of compounds that is used in this process is trivalent phosphites, already known as stabilizers in the plastic industry. The reaction rate of phosphites with some oxygen donors is high enough to compete with the oxygen addition to carbon-centered radicals. Figure 8 shows the interaction of the phosphite during the curing process. The phosphite moiety is oxidized stoichiometrically to a phosphate ion and its role is to produce the reactive radical that was previously deactivated by an oxygen molecule.
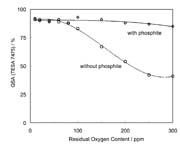
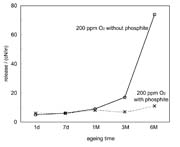
Figure 9 shows the influence of the oxygen concentration in the coating unit on the subsequent adhesion. Although 300 ppm of oxygen is present in the system, the phoshite yields >80% subsequent adhesion whereas without a stabilizer curing would not take place. These results show that it is easier to reach good curing conditions using our method. In addition, the production safety increases. Furthermore, using the stabilizer as illustrated in Figure 10 can reduce the aging of the laminate, thus allowing it to act as an "aging stabilizer." These results show that it is easier to reach good curing conditions using our method.
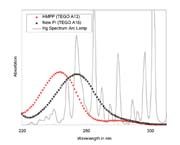
Many photoinitiators are available for use in this process. For silicone acrylates, 2-hydroxy ketones are recommended, as they have been proven to be highly efficient with the UV spectra of standard UV lamps (medium-pressure mercury lamps). Furthermore, they are often liquids, which is advantageous for blending with silicone acrylates. The most common photoinitiator of this class is 2-hydroxy-2-methyl-1-phenyl-1-propanone (HMPP, CAS No 7573-98-5).
HMPP is a widely available and well-investigated UV photoinitiator. Although HMPP and its resulting radicals blend with the silicone system, they are not hydrophobic molecules. Thus, they may not easily escape the solvent cage and may not be able to move freely within the silicone matrix to react with the acrylic groups. We therefore concentrated our investigations to find a more hydrophobic photoinitiator. This new photoinitiator is now available.
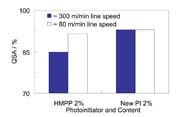
Even though the absorption of UV light is important, the yield of radical conversion from the exited state is even more important. Eventually, all these factors determine the cure-speed potential of a photoinitiator. To measure the cure speed, it is best to run a coating trial. To set up such a trial, one can increase machine speed or reduce the UV output to the critical stage, where the degree of cure is not fully developed. The degree of cure can be measured with the subsequent adhesion test. Figure 12 shows that more hydrophobic photoinitiator leads to better curing, especially at higher machine speed.
Conclusion
It is difficult to expect quantum leaps when it comes to the performance of UV silicone release coatings in this industry. However, the results presented above show that there is potential for the use of this environment-friendly technology.For more information on release coatings, contact Degussa Corp., e-mail info@tego-rc.com or visit http://www.tego-rc.com.
References
1 Crivello, J.V.; Feldmann-Krane, G.; Oestreich, S. US 2002/0193619.2 Oestreich, S.; Mueller, W. US 6365643.
3 Jachmann, J.; Weitemeyer, C.; Wewers, D. EP 0468 305.
4 Eckberg, R.P. EP 334 068.
5 Doehler, H.; Pomerin, J.; Brandt, M.; Hahmann, W. DE 102 328 28.5 US and EP patent pendant.
6 Doehler, H.; Pomerin, J.; Brandt, M.; Hahmann, W. DE 102 481 11.3 US and EP patent pendant.
7 Doehler, H. Munich Adhesiv an Finishing Symposium, 2002, Munich.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






