Advancing Adhesives: Bio-Based Succinic Acid Polyester Polyols
Sustainable building blocks provide multiple benefits for performance-driven TPUs, PUDs and coatings.
Bio-based succinic acid provides researchers and product developers a valuable and innovative sustainable platform chemical for differentiable, high-performance polyurethane (PU) materials. Bio-based succinic acid and its derivatives have demonstrated performance advantages in a variety of applications, including personal care products, non-phthalate plasticizers, and polymeric derivatives used in urethanes, polyesters, and alkyd resin technologies.
Figure 1 shows a schematic of the various synthetic pathways by which succinic acid can be transformed to diesters, diols, and high-molecular-weight polyesters and polyester polyols. Along with succinic acid, key succinate derivatives include polyester polyols,1,4 butane diol (BDO) and tetrahydrofuran (THF).
Yeast Fermentation Process and LCA
Since 2010, BioAmber has produced bio-based succinic acid at a demo facility in Bazancourt, France. With this facility, the company has been able to create and optimize a fermentation process that has been further developed into a cost-efficient process at a 30,000-ton facility in Sarnia, Ontario, Canada. This facility is currently in the commissioning phase and will start production in the second half of 2015. It will utilize an acidic yeast fermentation process to directly produce succinic acid without having to go through dibasic salt intermediates, thus saving time-intensive separation and purification process steps. Thus, consistent high-quality, polymerization-grade succinic acid is produced in a more sustainable and energy-efficient manner.
Independent third-party lifecycle analysis (LCA) has estimated that, when fully operational, the Sarnia manufacturing fermentation process can reduce carbon emission by up to 100% compared to petro-based adipic acid, and can reduce energy consumption by nearly 65%. This is the equivalent of saving over 200,000 tons per year of CO2 emissions or reducing GHG emission by the equivalent of 24 million gal of gasoline. These findings are shown in Figure 2 and are based on field-to-gate emission modeling.
However, even with the reduction of GHG emission and energy, the adoption of a new chemical building block like bio-based succinic acid over established petrochemical stalwarts (e.g., adipic acid) needs to offer demonstrable performance advantages to facilitate the adoption process. Over the last few years, it has been demonstrated that bio-based succinic acid can indeed bring differentiable, value-added performance to materials such as polyesters, TPU elastomers, and polyester and alkyd resins.
For example, in polyester polyols for polyurethanes, the C4 carbon chain of succinic acid can replace the C6 adipic acid to improve the solvent resistance and maintain excellent mechanical properties, including tensile strength, elongation, and abrasion resistance. In polyester resins for coatings, the C4 succinic acid can replace C6 adipic and aromatic acids to improve resin flexibility without significant loss of the glass-transition temperature (Tg) of the resin. This article provides a review of how succinic acid can be used to synthesize modified polyester polyols and bring advantages to polyurethane elastomers and coatings.1-3
Succinate Polyester Polyols
Polyester, polyether and polycarbonate polyols are key soft block structures used to influence the properties of polyurethane TPU and elastomers. A generalized polyurethane scheme is shown in Figure 3 for the reaction of a succinate polyol with 4,4’-MDI.
The polyester polyols enable formulators to generate a range of polyurethanes with diverse physical properties influenced by the choice of the diacids and diols (glycols) used to make the polyol. This soft segment can influence key properties such as Tg, melting point (Tm), and heat and tear resistance. Table 1 exemplifies general polyurethane trends; Table 2 shows some specific data based on different formulations.4
The synthesis and molecular weight control of polyester polyols follows the principles of condensation polymerization established by Carothers. The modified Carother’s equation shown in Equation 1 is for an off-stoichiometric ratio of acid to glycol using excess glycol. Using this relationship, it is possible to control the degree of polymerization and hence the hydroxyl number for the polyols:
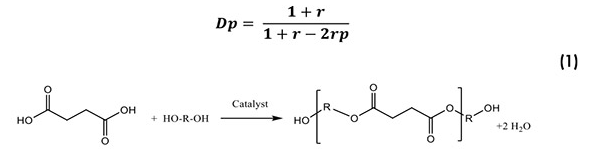
The physical properties of the succinate polyols are controlled by the type of glycol or glycol mixture reacted with the succinic acid. As a result of the structural influence of the polyol, it is possible make a range of polyester polyols with different Tg, Tm and viscosity values. Generally, succinate polyols will have a higher glass-transition temperature than adipates and may have a greater tendency to crystallize, especially if condensed with 1,4 butane diol (BDO) as the glycol. However, it is possible to obtain succinate polyester polyols with glass temperatures less than -50°C, as exemplified in Table 3. Table 3 also shows the tendency of butylene succinates to form crystalline solids and to have somewhat higher viscosity values than corresponding adipates. Interestingly, even SA-BDO polyols with mixed glycols were still solids at room temperature.
Computer modeling provided a 3D image showing how the glycol or mixed glycol influences the physical properties of the polyol and helps to explain the Tg, Tm and viscosity behavior of the data listed in Table 3. From this modeling, it is apparent that when an even-number diol, such as BDO, is used with succinic acid, the carbonyl ester groups orientate on the opposite side of the polyol chain to minimize energy and enhance polarity, which allows for stronger inter-chain alignment and hence the tendency to crystallize. The proton, carbon and oxygen atoms are distributed spatially so that the largest substituents are in anti-conformation, with dihedral angles between the largest substituents of 180° along the chain.
The molecular arrangement of SA-BDO shows increased linearity; this linearity can explain why polyols with SA-BDO combinations tend to form waxy solids even in mixed glycol systems and why, for instance, SA-BDO polyester homopolyomer forms the highest-melting aliphatic polyester polyol, with a melting point of about 108-112°C. Moreover, when incorporated into the soft block of the TPU, the SA-BDO sequence forms a highly regular, more rigid structure, which can partially explain the tendency of TPUs made with this polyol to exhibit higher Tg, hardness, abrasion resistance and toughness.
However, odd-number glycols (such as PDO) force the carbonyls to the same side of the polyester chain and introduce a kink along the axis of the polyester chain. The odd-numbered diol creates what appears as a wave down the chain axis and accounts for the more amorphous nature of the polyols and the tendency of this material to form TPUs with lower moduli and higher flexibility than the corresponding polyols based on BDO.
Polyol Hydrolytic Stability
To further evaluate the hydrolytic stability of the SA-polyols, a series of the polyester polyols was exposed to 70°C and 95% relative humidity (RH) for 20 days. The hydroxyl number as a function of time was analyzed to determine if hydrolysis of the ester linkage could be detected by NMR. It was theorized that if, when exposed to these conditions, the polyesters degraded, the hydroxyl number would increase as the esters groups hydrolyzed. Moreover, we considered the polyol group, when not part of a PU matrix, to be an excellent proxy for the ultimate PU stability since the ester groups could not be stabilized by the hard block domain of the PU.
The changes in hydroxyl values were determined by NMR. The changes of the normalized hydroxyl numbers are plotted in Figure 5 for a series of SA and AA polyols. Very little change in the hydroxyl values was detected by NMR. All values are within a margin of ± 5 OH units, suggesting very little change in the number of repeat units of the polyester polyols. Nonetheless, these data indicate the SA polyols have similar hydrolytic stability compared to similar adipates. Additional hydrolysis testing under more aggressive conditions is in progress.
Succinate Polyester Polyols in PUD Coatings
PU coatings represent a significant application opportunity for bio-based succinate polyester polyols and are an area of active interest for new technical and product development. BioAmber and researchers at North Dakota State University recently studied hexane diol succinates (SA-HD) in combination with aliphatic isocyanates HMDI for metal coatings. This study was done in parallel with an application study with Stahl International for PU coatings on textiles and flooring; some of these findings will be presented in more detail at a future conference.1
Two polyester polyols with an average molecular weight of 1,000 and 2,000 g/mol based on succinic acid and 1,6-hexanediol were synthesized and studied for use in making polyurethane dispersions and coatings. Similar polyols were prepared from 1,6-hexanediol and adipic acid, the most common aliphatic diacid used, to synthesize polyester polyols as control samples. The abbreviated names of the polyester polyol consist of the name of the acid, where SA stands for succinic acid, AA for adipic acid and the diol (HD) stands for hexane diol, followed by the target average molecular weight of the polyester. The scheme of the reaction is shown in Figure 6 (p. 40). In the presence of dibutyltin oxide, the target acid number (< 2 mg KOH/g) was reached in an 8-10 hour period for all polyesters. All synthesized polyester polyols are white solids at room temperature and transform into clear light-yellow liquids above ~ 50°C.
All polyester polyols exhibit melting endotherms at 44-54°C as determined by differential scanning calorimetry (DSC). Melting points of the polyester polyols are listed in Table 3. Succinates and adipates polyols with the same molecular weight have similar melting points; however, increasing the molecular weight from 1,000 to 2,000 g/mol causes a slight increase in the Tm for both types of polyester polyols.1
Characteristics of PUDs Made Using Polyester Polyols and Coatings
Four polyurethane dispersions were prepared from the polyester polyols by way of an N-methyl pyrrolidone (NMP) process (see Figure 7). The synthesis involves the formation of a carboxyl-containing isocyanate functional prepolymer, followed by neutralization of the carboxylic acid groups with amine, dispersion in water, and chain extension to obtain the aqueous PUD. The first step of the process included reacting a polyester polyol and dimethylolpropionic acid (DMPA) with H12MDI in the presence of DBTDL. The free carboxyl groups of the prepolymer were neutralized with triethylamine, and the prepolymer was dispersed in water and reacted with ethylenediamine, a chain extender, to form the final PUD.
The three PUD formulations were used to make coatings on steel and aluminum substrates to evaluate the performance of succinate-based PUDs vs. adipate-based ones. After drying, coated panels were left for one week for the hydrogen bonding to develop in the PU films. The characterization results of the coated samples are summarized in Tables 5 and 6 (steel substrate and aluminum substrate, respectively).
As evidenced by reverse impact hardness and mandrel bend tests, all of the PU formed very flexible coatings on both the steel and aluminum substrates. In the conical mandrel bend test, no signs of cracks were observed at the smallest mandrel diameter for all coatings. In the reverse impact test, all coatings scored above the upper measurement limit of the instrument. Both succinate-based polyurethane coatings have the same pencil hardness rating as the adipate-based coatings. The difference in pencil hardness values reported on steel and aluminum could be explained by the differences in coating-substrate interactions on the two types of panels. Both succinate and adipate-based coatings have the highest possible crosshatch adhesion rating of 5B. Coatings made from SA-HD-2000-based PU have an adhesion rating of 5B on steel and 4B on aluminum, perhaps indicating that the affinity of this type of PU to steel is slightly better than to aluminum.
Regardless of the substrate, König hardness values measured for the PU based on SA-HD-2000 were much lower than the measured value for SA-HD-1000. Increasing molecular weight of the succinate-based polyester polyol causes an increase in the soft segment content of the polyurethane, which, in turn, leads to a softer polymer. This is explained by the longer soft block of the SA-HD-2000 compared to the SA-HD-1000 essentially diluting the H-bonding sites of the urethane linkage and therefore softening the polymer.
As highlighted by the red boxes in Tables 5 and 6, the lower König hardness of the SA-HD 2000 coating did not impact the solvent resistance to MEK rubs compared to the harder AA-HD polyol. This performance attribute shows a potential benefit of the succinate polyols to potentially enable coatings with improved solvent resistance despite the fact that the coating is softer. This actually suggests the SA-based polyols may enable formulators to break a performance tradeoff by enabling softer coatings that still have improved solvent resistance.
Mechanical Properties and Hydrolytic Stability of PUD Films
PUD films were subject to tensile testing to evaluate mechanical properties. The representative stress-strain curves for each PU are shown in Figure 8;
Table 7 shows the ultimate stress and strain properties. The three PUs demonstrated very different behaviors under load. While stress-strain curves for SA-HD-1000- and AA-HD-1000-based polyurethanes are very close in the elastic region (implying similar values of the Young’s modulus), the SA-HDO-1000 has a higher stress at a given strain compared to the AA-HDO 1000. This increase in modulus is consistent with the shorter C4-succinic acid segment of the polyol increasing the relative stiffness of the soft block relative to similar adipate analogue of similar molecular weight. As anticipated, the softer SA-2000 has lower stress values than either of the 1000-molecular-weight PEPs as a result of the lower hard block content of the segmented PU.
In order to test the relative hydrolytic stability of the succinate-based PU coatings vs. the adipate-based PU coatings, a set of free-standing films was placed in an environmental chamber saturated with water vapor at 60°C. The films were removed from the chamber after 48 and 120 hours of exposure, and tested for mechanical properties. The changes in the tensile values at different strains are shown in Figure 7 for the initial and five-day exposure times.
The data in Table 8 exemplify two features of the SA-polyols compared to the adipic acid polyols: the succinicate polyols have hydrolytic stability at least as good as adipate polyols under these conditions, and the PU made with the lower-molecular-weight polyol SA-1000 is much stiffer than the corresponding PU made with AA-HD 1000. However, the PU made with the higher molecular weight polyol SA-HD 2000 provided for a PU coating with excellent mechanical properties, potentially exceeding those of the AA-HD 1000 system but with improved solvent resistance (see Tables 5 and 6).
In fact, dynamic mechanical analysis (DMA) data shown in Figure 9 compares the storage modulus of these films. The G’ moduli values clearly show the higher stiffness of the PU film made with SA-HD 1000 compared to AA-HD 1000. In fact, PU films made with the SA-HD 2000 have dynamic moduli values more similar to PUs made with AA-HD 1000 than the SA-HD 1000. This suggests that, when considering a bio-based SA-polyol in place of the AA-polyol, a higher-molecular SA polyol may be preferred to optimize the PU properties such as hardness, strength, elongation, and durability.
Flexible Formulation
These data exemplify the formulation flexibility of bio-based succinic acid and its use as a key building block in polyester polyols for PUs—especially for PU coatings. The succinic acid building block enables PUs with greatly improved solvent and abrasion resistances, and can produce PUs with greater stiffness and higher moduli values.
There is clearly some positive anomalous behavior that the formulator can exploit by using bio-based succinate polyols. Mainly, these bio-based systems provide improved hardness and solvent and abrasion resistance for more durable PUs. These modified properties open up the formulation window for PU systems that are not only good for the environment, but are value-added formulations tools for PUs.
For more information, contact the lead author at bill.coggio@bio-amber.com or visit www.bio-amber.com.
Author’s Acknowledgement
The lead author wishes to acknowledge the technical support and collaboration of Ivan Hevus, Ph.D., Prof. Dean Webster, Kyle Kingsley, and Ken Croes at the North Dakota State University; Niek Zweep, Ph.D., of Stahl International; and Alan Schrock, Ph.D., Baylen Thompson, and Kenneth Ulrich of the University of West Florida for their diligence and excellent collaborations, especially in the work cited in References 1 and 2, respectively. This information and technical advice (whether verbal, in writing or by way of trials) are given in good faith but without warranty, and this also applies where proprietary rights of third parties are involved. Our advice does not release you from the obligation to verify the information currently provided (especially that contained in our safety data and technical information sheets) and to test our products as to their suitability for the intended processes and uses. The application, use and processing of our products and the products manufactured by you on the basis of our technical advice are beyond our control and, therefore, entirely your own responsibility.
Editor’s note: This article is based on a paper presented at the Center for Polyurethanes Industry’s 2014 Technical Conference.
References
1. Coggio, W.D., Mullen, T., Hevus, I., Croes, K., Kingsley, K., Webster, D.C., Zweep, N., “Bio-Based Succinic Acid: A Versatile Building Block for Performance Driven PUD and Coatings,” presented at American Coatings Conference Session 2, Biobased Coatings, Atlanta, April 2014. A second phase of the PUD studies also conducted at NDU were published and presented at Waterborne Conference, University of Southern Mississippi, New Orleans, La., February 12, 2015.
2. Coggio, W.D., Schrock, A., Thompson, B., Ulrich, K., “Modified Polyester Polyol Succinates Derived from Bio-Based Succinic Acid and Branched Glycols: Influence of Glycol Structure on Tg, Tm and Process Viscosity,” presented at UTECH-NA, Charlotte, N.C., June 4, 2014.
3. Szycher, M., Handbook of Polyurethanes, CRC Press, 1999.
4. Table 2, reproduced from Reference 2, page 5. The original sources cited in this table are the references from the article cited in Reference 2.
5. Mullen, T.J., Webster, D.C., Nasrullah, M.J., Eidenschink, N.T., “Bio-Succinate Polyesters for Thermoplastic Polyurethanes Enabling Sustainable New Products with Performance Inspired by Nature,” Council for Polyurethane Industry (CPI) Conference, Atlanta, September 2012.
6. Miller, R., Janssen, R., Theunissen, L., “Evaluating the Properties of Performance of Susterra® 1,3 Propanediol and Biosuccinium™ Sustainable Succinic Acid in TPU Applications,” Council for Polyurethane Industry (CPI) Conference, Atlanta, September 2012.
7. Janssen, R., Theunissen, L., “Evaluating the Properties of Performance of Biosuccinium™ Sustainable Succinic Acid Based Copolyester Polyols in TPU Applications,” Council for Polyurethane Industry (CPI) Conference, Phoenix, Ariz., September 2013.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




