Advancing Adhesives
Underwater Bonding
A new “biomimetic” glue can provide high-strength bonding under water.
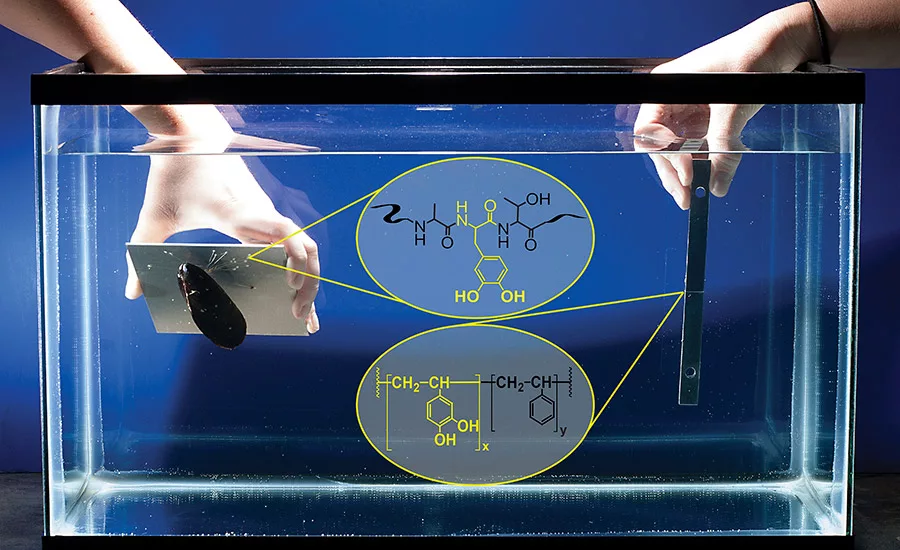
When it comes to underwater adhesion, shellfish are the true experts. Mussels, barnacles and oysters attach to rocks with apparent ease. Yet our man-made glues often fail when trying to stick in wet environments. “Our current adhesives are terrible at wet bonding, yet marine biology solved this problem eons ago,” said Jonathan Wilker, a professor of chemistry and materials engineering at Purdue University. “Mussels, barnacles and oysters attach to rocks with apparent ease. In order to develop new materials able to bind within harsh environments, we made a biomimetic polymer that is modeled after the adhesive proteins of mussels.”
New findings, funded by the Office of Naval Research, showed that the bio-based glue performed better than 10 commercial adhesives when used to bond polished aluminum. When compared with the five strongest commercial glues included in the study, the new adhesive performed better when bonding wood, Teflon™ and polished aluminum. It was the only adhesive of those tested that worked with wood, and it far out-performed the other adhesives when used to join Teflon. The findings were detailed in a research paper published online and in a print issue of the journal ACS Applied Materials and Interfaces.
Mussel Proteins
Mussels extend hair-like fibers that attach to surfaces using plaques of adhesive. Proteins in the glue contain the amino acid DOPA, which harbors the chemistry needed to provide strength and adhesion. Purdue researchers have now inserted this chemistry of mussel proteins into a biomimetic polymer called poly(catechol-styrene), creating an adhesive by harnessing the chemistry of compounds called catechols, which are contained in DOPA.
“We are focusing on catechols given that the animals use this type of chemistry so successfully,” Wilker said. “Poly(catechol-styrene) is looking to be, possibly, one of the strongest underwater adhesives found to date.”
While most adhesives interact with water instead of sticking to surfaces, the catechol groups may have a special talent for “drilling down” through surface waters in order to bind onto surfaces, according to Wilker. The series of underwater bond tests were performed in tanks of artificial seawater.
Getting Wet
“These findings are helping to reveal which aspects of mussel adhesion are most important when managing attachment within their wet and salty environment,” Wilker said. “All that is needed for high-strength bonding underwater appears to be a catechol-containing polymer.”
Surprisingly, the new adhesive also proved to be about 17 times stronger than the natural adhesive produced by mussels. “In biomimetics, where you try to make synthetic versions of natural materials and compounds, you almost never can achieve performance as good as the natural system,” Wilker said.
One explanation might be that the animals have evolved to produce adhesives that are only as strong as they need to be for their specific biological requirements. The natural glues might be designed to give way when the animals are hunted by predators, breaking off when pulled from a surface instead of causing injury to internal tissues. Future research will include work to test the adhesive under real-world conditions.
“We have shown that this adhesive system works quite well within controlled laboratory conditions. In the future, we want to move on to more practical applications in the real world,” Wilker said. ASI
For more information, contact the author at wilker@purdue.edu or visit www.purdue.edu.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





